The Psychedelic News Feed
October 27 - November 02, 2025
This is an archived version of our Psychedelic News Feed. Explore the live Feed and receive a weekly digest to your inbox by joining our free newsletter:
Learn more about and subscribe to our Pα+ program to receive lots, lots more. ∎
A Note from the Editor
It’s been an incredibly active start to the fourth and final quarter of 2025.
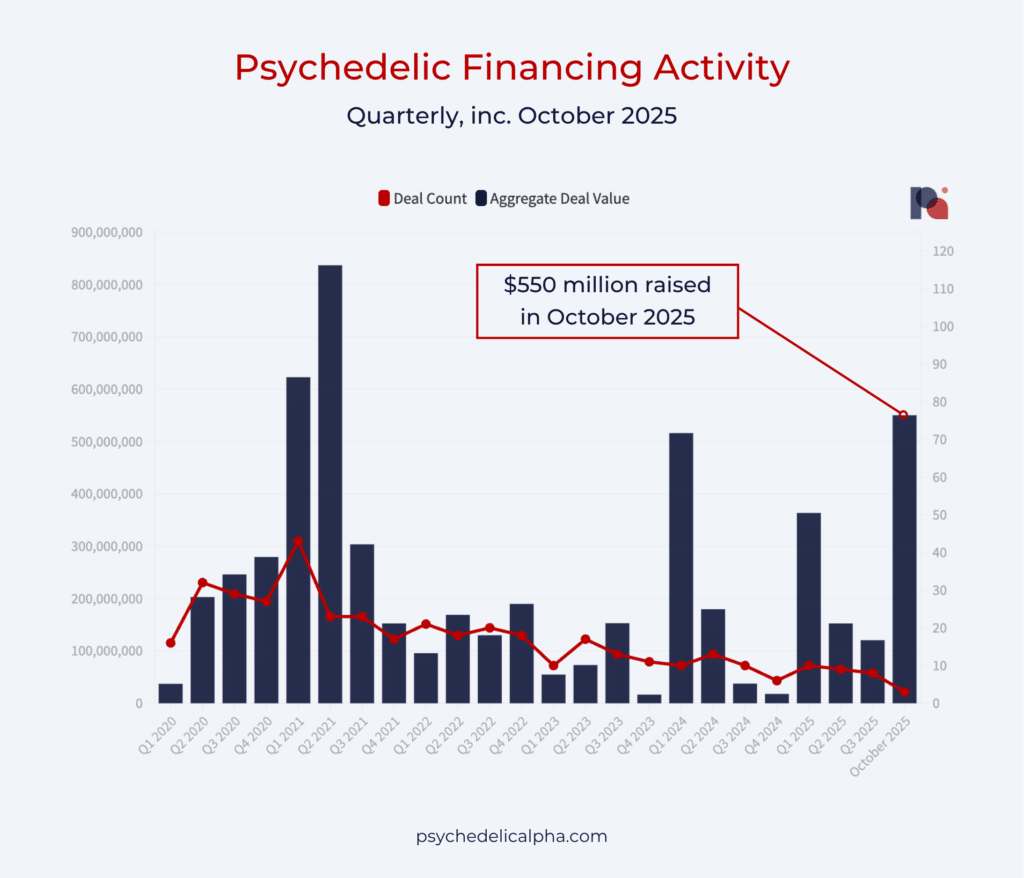
In October, funding for psychedelics companies appears to have caught up with the positive sentiment exhibited in our Q3 Psychedelic Investor Survey. More than half a billion dollars was raised across three rounds alone last month (by atai, Cybin, and MindMed), meaning Q4 2025 will see the largest inflows to the space since mid-2021. There are still two months left of the quarter, meaning the heady days of 2021 could still be topped.
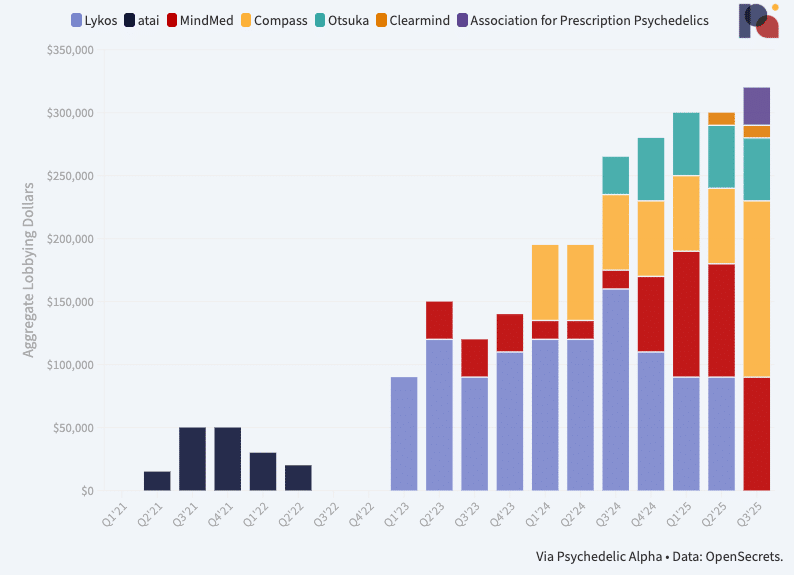
On the lobbying front, too, spend is ramping up. Our Psychedelics Federal Lobbying Tracker shows that psychedelic drug developers spent more than ever on U.S. federal lobbying in Q3, though the aggregate reported spend remains relatively modest at just north of $300k over the three-month period. Interestingly, Lykos Therapeutics has ceased its formal spend on federal lobbying, while Compass Pathways has more than doubled its own. What’s more, the Association for Prescription Psychedelics (which represents most of the late-stage developers in the field) began spending cash on lobbying activities in Q3, according to filings. (Pα+ subscribers can read our analysis of the latest data.)
On Friday, the Australian Government’s Department of Veterans’ Affairs announced that it has decided to fund psychedelic assisted therapy for veterans who meet certain criteria. It’s the latest in a string of psychedelics-related headlines from down under, which we will be covering in more detail very soon.
In the 212th Issue of our Psychedelic Bulletin, we looked at several other stories, including Delix Therapeutics’ efficacy signal from a small open-label study of its lead neuroplastogen and NRx Pharmaceuticals’ efforts to have FDA ban its competitors’ ketamine products.
Below, you will find your Psychedelic News Feed, a one-stop digest for the latest coverage of psychedelics business, policy, research and beyond.
Josh Hardman
Founder & Editor
New: Psychedelics Federal Lobbying Tracker ↗ Psychedelic Alpha
Here, we share several charts that visualise the extent of psychedelics organisations’ lobbying activities at the federal level, at least according to filings. ∎
Q3’25: Psychedelic Lobbying Update (Oct 29) ↗ Psychedelic Alpha
Psychedelic Bulletin 212 (Oct 31) ↗ Psychedelic Alpha
- Delix Therapeutics Posts Early Efficacy Signal for ‘Non-Hallucinogenic’ Neuroplastogen as FDA Clears At-Home Phase II
- NRx Pharmaceuticals Wants FDA to Ban Its Competitors’ Ketamine
- Cybin Trades Toxic Financing for Top Tier Investors in $175M Raise
- and more…
Psychedelics muddy fMRI results: Q&A with Adam Bauer and Jonah Padawer-Curry (Oct 29) ↗ The Transmitter
Inside NZ’s first legal magic mushroom trip: ‘I felt big emotions to my core’ (Oct 29) ↗ 1 News
Could psychedelic drugs be the future of medicine? | The Big Story (Oct 29) ↗ CityNews
Delix Therapeutics Announces Positive Efficacy Data for DLX-001 (Zalsupindole) and FDA Clearance of Phase II Trial Design Featuring At‑Home Administration (Oct 28) ↗ Press Release
In Waves and War review – Navy Seals battle PTSD with psychedelic therapy (Oct 31) ↗ The Guardian
The Swiss Wrote the Psychedelic Playbook; Now Europe Follows (Oct 31) ↗ Medscape
After Oct. 7, Jews seek healing from kabbalah-informed psychedelic retreats (Oct 30) ↗ AP News
Your guide to Psychedelic-Assisted Therapy (Oct 29) ↗ University of Cambridge
FDA criticism of MDMA-assisted therapy is an opportunity for psychedelic medicine (Oct 30) ↗ STAT
This is an archived version of our Psychedelic News Feed. Explore the live Feed and receive a weekly digest to your inbox by joining our free newsletter:
Learn more about and subscribe to our Pα+ program to receive lots, lots more. ∎