As Gilgamesh Pharmaceuticals is set to share positive results from its Phase 2a study of GM-2505 (aka bretisilocin, a serotonergic psychedelic) in major depressive disorder later today at ASCP, we speak with the company’s Chief Strategy and Development Officer Jorge Quiroz and Chief Operating Officer Yoni Falkson to dig a little deeper into the study, its results, and the company’s plans for the candidate.
***
Reporting by Josh Hardman
This morning, Gilgamesh Pharmaceuticals shared impressive topline data from a Phase 2a study of GM-2505, a substituted tryptamine derivative, in major depressive disorder (MDD).
We broke the news of this data earlier in the month, when we spotted a high-level sketch of the results in the American Society of Clinical Psychopharmacology’s (ASCP) agenda. The company will present a more detailed topline readout at that meeting this afternoon, followed by poster sessions tomorrow and on Thursday.
In this coverage, we take a closer look and speak with company execs.
The trial evaluated the drug in 40 patients, randomised to receive either 10 mg or 1 mg (“a low-dose psychoactive comparator”, according to the company) of GM-2505 on Day 1, followed by a 15 mg dose of GM-2505 on Day 15 across both arms. The drug was administered intravenously.
In terms of the model of non-pharmacological support employed in the trial, Gilgamesh told us that it only used “psychological support/education and safety monitoring.” “Psychotherapy credentials were not required for facilitators in this study”, a company executive said.
The company would not disclose the number of these pre- and post- sessions.
The headline finding is very positive, with a rapid and sustained antidepressant effect observed. At 24 hours, the study reports a -18.5 point MADRS change from baseline in the 10 mg group, which grew to a -21.6 point change at Day 14. That’s 9.6 points greater than the reduction seen in the 1 mg group, demonstrating convincing separation.
What’s more, the company reports a -28.0 point change at Day 29 in the 10 mg + 15 mg arm, with a change of -25.1 at Day 74 suggesting the effect is durable [1], at least in this small study. In terms of MADRS remission rates (defined as a total score of ≤10), that translates to 70% at Day 14 and 94% at Day 29.
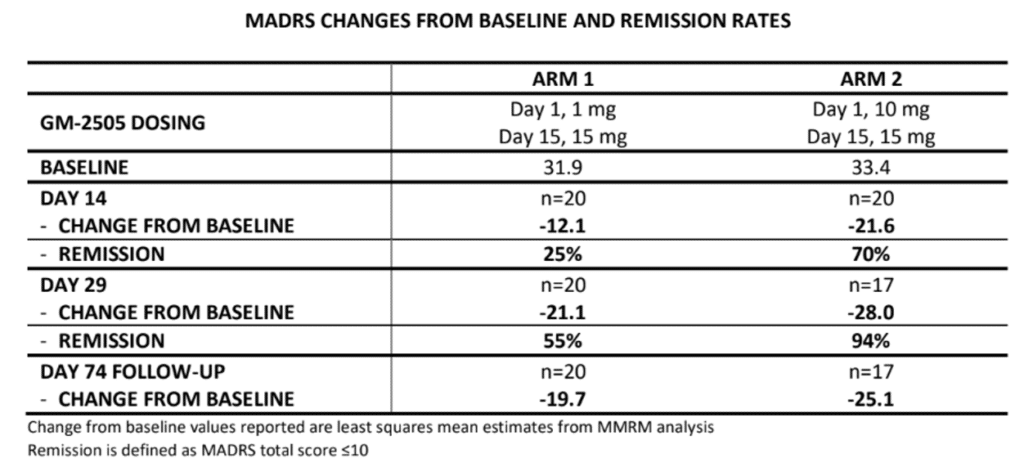
Speaking to Psychedelic Alpha, Gilgamesh Chief Strategy and Development Officer Jorge Quiroz pointed out that after day 29 in the second arm, some patients are almost close to zero on the MADRS. That explains “why we have this unbelievable remission rate”, he told us.
Quiroz described these results, and the effect size of 1.0, as “extraordinary”. For context, conventional antidepressants tend to have an effect size in the region of 0.3.
To continue reading, please log in or join Pα+…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue


