A recurrent theme in our bulletins is the crowded nature of drug development efforts surrounding certain molecules and indications. This week, using MindMed’s Phase I DMT trial announcement as the impetus, we take a brief look at other companies seeking to develop DMT-based therapeutics.
Yesterday, Field Trip became the latest psychedelics company to list on the NASDAQ, just over a month after atai’s IPO. Elsewhere, COMPASS Pathways appointed Professor Guy Goodwin as Chief Medical Officer.
Psychedelic Sector News
MindMed Initiates Phase I Trial of IV DMT
Earlier this week, MindMed announced the initiation of a Phase I clinical trial of DMT (NCT04353024), the naturally occurring psychedelic that is commonly consumed in the form of ayahuasca.
The investigator-initiated study will consist of 30 healthy participants and, like other MindMed studies, be led by Dr. Matthias Liechti at University Hospital Basel. The trial aims to assess the subjective and physical effects induced by different intravenous (IV) DMT administration schedules, seemingly inspired by previous work done by Andrew Gallimore and Rick Strassman. This includes testing whether a dose administered before a prolonged IV infusion, known as a bolus or loading dose, is able to safely and quickly induce an altered state.
As is explained in our Drug Development Tracker, MindMed’s target indication for DMT remains unknown. This is not surprising, given the amount of competition surrounding the development of DMT-based therapeutics (more on that below).
The main draw of DMT versus other psychedelics is its rapid onset and short-acting nature. MindMed presumably hopes that by administering it intravenously, it can prolong the short-lived experience and potentially gain greater control over the patient’s experience.
Dr. Miri Halperin Wernli, Executive President of MindMed, claimed that, “no study has validly determined the elimination half-life of DMT or other pharmacokinetic parameters.” However, this doesn’t guarantee that MindMed will be the first to obtain such data.
MindMed Not Alone in Trialing DMT
London-based Small Pharma has been dosing for its Phase I/IIa clinical trial since February 2021, and aims to complete the trial during the latter half of 2022 (NCT04673383). The company’s Phase I trial involves 32 healthy subjects, a similar number to MindMed’s, and will target Major Depressive Disorder.
In another similarity to MindMed’s study, Small Pharma’s lead candidate (SPL026) is delivered via intravenous injection. The company also has an extended duration IV in its pipeline (SPL028), along with other candidates.
Small Pharma is also exploring deuteration, and expects the peak subjective experience of an individual on DMT to be extended when DMT is partially or fully deuterated. Along with potential increases to the duration of peak subjective experiences and metabolic stability, deuteration also increases the patentability of molecules. In fact, Small Pharma secured its first patent covering deuterated DMT back in May (read our bulletin for coverage of that patent, as well as a primer on deuteration).
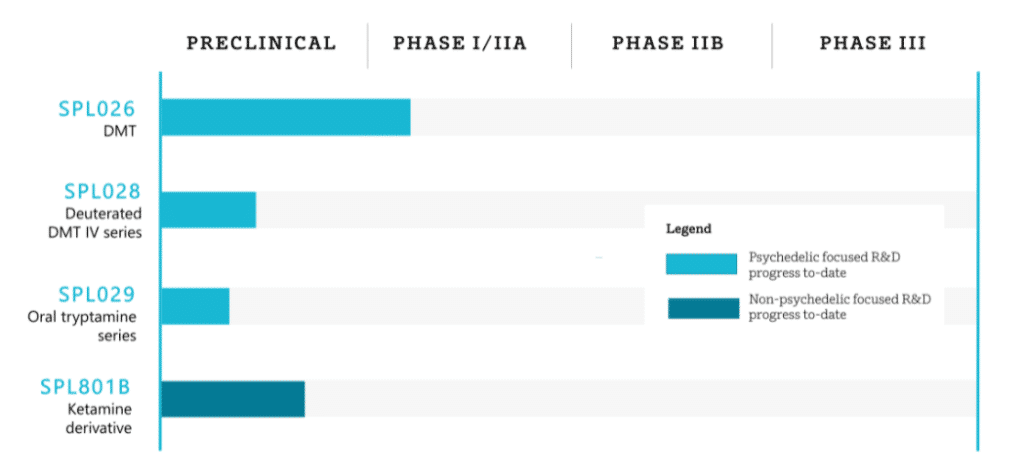
Other companies working on DMT include atai Life Sciences and Entheon Biomedical. Entheon’s lead drug development programme is IV DMT for the treatment of undisclosed substance use disorders. The company hopes to begin a Phase I study to assess the pharmacokinetics and pharmacodynamics of DMT fumarate in late 2021. However, given that much larger companies are now running very similar trials, one may expect Entheon to shift focus.
In fact, a pivot already seems underway, with the company deepening its foray into genetic testing to understand the risk profile of an individual prior to the administration of a psychedelic. Earlier this year, the company acquired psychedelics genetic test kit company HaluGen, and tests went on sale in June. Psilocybin Alpha understands that the validity, specificity or utility of such tests has not been studied rigorously, if at all. Today, Entheon announced the acquisition of Lobo Genetics, a “genetic screening platform technology” for personalised insights into one’s response to cannabis products. As with HaluGen, Lobo is a primarily direct-to-consumer platform, and has a virtually identical website.
Viridia Life Sciences, a programme of atai Life Sciences, is also investigating DMT. However, details on this programme are sparse, though Viridia CEO Glenn Short told Benzinga that they aim to “slow the onset and lengthen the duration of the [DMT] experience slightly, so that the psychedelic onset is much more gradual and overall more gentle and agreeable to the patient.” Viridia will seek to target treatment resistant depression.
As we have commented before, there is increasing competition across molecules, indications, and value chain segments. As such, we should expect to continue seeing smaller companies pivot accordingly.
Field Trip Lists on NASDAQ
Yesterday, Field Trip Health began trading on the NASDAQ Global Select Market, becoming the fifth psychedelics company to trade on NASDAQ. For now, the company offers ketamine treatments across six locations, though its Amsterdam outpost is able to offer psilocybin via truffles.
Trading was relatively muted, with FTRP closing down 3% yesterday.
COMPASS Pathways Announces Chief Medical Officer
Yesterday, London-based COMPASS Pathways announced the appointment of Professor Guy Goodwin to the position of Chief Medical Officer. Goodwin is a prominent psychiatrist, who formerly headed the University of Oxford’s Department of Psychiatry and held the position of President of the British Association for Psychopharmacology, among other titles.
At present, Guy Goodwin is Medical Director at P1vital, a CRO-style clinical research company seeking to make CNS treatment development more efficient through the employment of innovative technologies like biomarkers and digital solutions. The Oxford-based company has also developed software-based interventions such as video games, somewhat reminiscent of UCSF’s Neuroscape centre under the leadership of Adam Gazzaley.
Commenting on his appointment, Goodwin said: “I have been working with COMPASS in an advisory capacity since the beginning of the phase IIb clinical trial of psilocybin therapy for treatment-resistant depression. I am now delighted to be taking a more active role in bringing this innovative therapy to patients who desperately need new options in psychiatry.”
Toward the end of the press release, the company announced that Piers Morgan (no, not that Piers Morgan) will be leaving his post as Chief Financial Officer towards the end of the year.
Other Headlines
- Awakn Life Sciences lists on OTC Market under ticker symbol AWKNF;
- Cybin to commence trading on NYSE American August 5th; announces size and pricing of public offering ($30m, $3.40 per share);
- Filament Health granted amendment to Health Canada licence;
- Levitee Labs acquires telemedicine platform, 3 pharmacies, and 5 addiction clinics;
- Numinus closes Q3 2021 with $63.2m cash position;
- Silo Pharma awarded notice of allowance for homing peptide patent;
- Tryp Therapeutics appoints Robin Carhart-Harris as Chairman of Scientific Advisory Board.
Weekend Reading
The Sydney Morning Herald Publishes Long Read on Psychedelic Therapy
This morning, the Sydney Morning Herald published a long read on psychedelics as part of its Explainer series.
Commenting on the renaissance in psychedelics research, the piece asks: What are these studies hoping to find? Could these mind-altering drugs be the long-sought answer to alleviating suffering caused by mental illnesses where other treatments have failed? Do they reveal the secrets of the universe? And what are the risks?
AOC Once Again Advocates for Psychedelic Drug Research
Alexandria Ocasio-Cortez was once again defeated when she forwarded an amendment to remove barriers to psychedelic research. While the defeat was certainly convincing (285-140), it was narrower than the last time the amendment was put to a vote in 2019, where it was defeated 331-91. Times, and views, are clearly changing.
It is ridiculous that Congress upholds War on Drugs-era barriers on federal research into substances like psilocybin, ibogaine,& MDMA when early results are indicating major promise in treating PTSD, addiction,& more.
— Alexandria Ocasio-Cortez (@AOC) July 22, 2021
I’m trying (again) to lift them so we can pursue the science. https://t.co/e87U3UYhUS
A piece in Inverse explores why AOC is an advocate for such research.
Weekly Bulletins
Join our newsletter to have our Weekly Bulletin delivered to your inbox every Friday evening. We summarise the week’s most important developments and share our Weekend Reading suggestions.
Live Updates
Join us on Twitter for the latest news and analysis.
Other Channels
You can also find us on LinkedIn, Instragram, and Facebook.


