Your Brief
A peer-reviewed study shows that MDMA-assisted psychotherapy is cost-effective when compared to existing treatments for PTSD.
The magnitude of this saving is expected to be in the range of $103.2 million over 30 years per 1,000 patients; with breakeven occurring at just over 3 years.
In many countries, these savings would benefit public healthcare systems or private insurance companies.
If coupled with efficacy data, which should be forthcoming in MAPS’ clinical trials, these cost savings could build a strong case for public healthcare systems and insurance companies to provide MDMA-assisted psychotherapy in the future.
It should be noted that the estimates are likely conservative, as they don’t take into account other monetisable benefits including increased productivity and lower disability payments that result from effective treatment of PTSD.
Full news release…
Healthcare cost savings of MDMA-assisted psychotherapy for PTSD estimated to be greater than $103.2 million with a return of 5,553 Quality of Life Years per 1,000 patients
SANTA CRUZ, Calif., Oct. 26, 2020 /PRNewswire/ — A peer-reviewed study published in the research journal PLOS ONE demonstrates that MDMA-assisted psychotherapy is remarkably cost-effective when compared to currently available treatments for posttraumatic stress disorder (PTSD). It is estimated that public healthcare payers or private insurers making MDMA-assisted psychotherapy available to 1,000 patients would reduce general and mental health care costs by $103.2 million over 30 years.
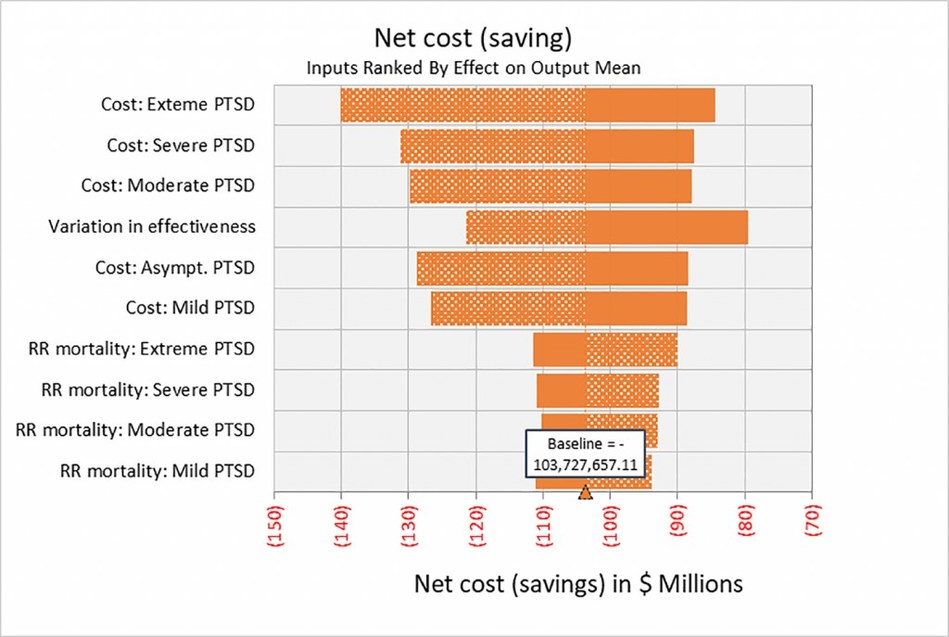
Lead author Elliot Marseille, Dr.P.H., M.P.P., elaborates, “MDMA-assisted psychotherapy is conducted by a licensed psychologist and trained clinician over the course of twelve sessions. The cost is not inconsiderable, but in just over three years, healthcare providers will break even on the costs of mental health and general medical care. These estimates are promising yet likely too conservative: the study did not measure the value of increased productivity or lower disability payments as patients recover and is constrained by the limited data on the long-term trajectory of PTSD. Further research will be needed to determine the full financial, personal, and societal benefits of MDMA-assisted psychotherapy for PTSD.”
Berra Yazar-Klosinski, Ph.D., Head of Research Development and Regulatory Affairs for MAPS Public Benefit Corporation and co-author, notes, “A growing body of evidence suggests that MDMA-assisted psychotherapy may be more effective than currently available treatments for PTSD, a notoriously difficult-to-treat condition. Previous research has focused on safety and efficacy and indicates statistically significant improvements over psychotherapy with a control, demonstrating reduction in symptoms for 82% of participants. This study should compel healthcare providers to include MDMA-assisted psychotherapy as a covered treatment for PTSD following FDA approval.”
Rick Doblin, Ph.D., Executive Director of MAPS and a study co-author, states, “The profound personal toll of PTSD can include deterioration in physical health, relationships, and ability to participate in social activities along with the anxiety, insomnia, and suicidal ideation that mark the condition. By demonstrating a return of an average of 5.5 quality-adjusted life-years over 30 years, we have shown that MDMA-assisted psychotherapy has the potential to reduce more than the personal burden of PTSD, contributing to improved health outcomes and reduced healthcare burdens for payers and providers.”
The cost-effectiveness of MDMA-assisted psychotherapy from the U.S. healthcare payers’ perspective was constructed with a decision-analytic Markov model to portray the costs and health benefits of treating patients with chronic, severe, or extreme, treatment-resistant PTSD. Efficacy was based on the pooled results of six randomized controlled trials with the 105 subjects who participated in Phase 2 trials and a four-year follow-up of 19 of those subjects. The model calculates expected medical costs, mortality, quality-adjusted life-years, and incremental cost-effectiveness ratio over 30 years.
NOTE
The safety and efficacy of MDMA-assisted psychotherapy is currently under investigation. This treatment has not yet been approved by the FDA, does not work for everyone, and carries risks even in therapeutic settings.
Disclaimer
The news site hosting this press release is not associated with Multidisciplinary Association for Psychedelic Studies (MAPS). It is merely publishing a press release announcement submitted by a company, without any stated or implied endorsement of the treatment, product or service.
About Multidisciplinary Association for Psychedelic Studies
ABOUT MAPS
Founded in 1986, MAPS is a 501(c)(3) non-profit research and educational organization that develops medical, legal, and cultural contexts for people to benefit from the careful uses of psychedelics. Since its founding, MAPS has raised over $100 million for psychedelic therapy and medical research and education. For more information, visit maps.org.
ABOUT MAPS PUBLIC BENEFIT CORPORATION (MAPS PBC)
MAPS Public Benefit Corporation (MAPS PBC) catalyzes healing and well-being through psychedelic drug development, therapist training programs, and sales of prescription psychedelics while prioritizing public benefit above profit. Founded in 2014, MAPS PBC is a wholly owned subsidiary of the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501(c)(3) non-profit organization. More information about the organization is available at mapspublicbenefit.com.
Press Contact
Betty Aldworth
202-489-2222
https://maps.org/mediarequest
SOURCE: MAPS


