Reporting by our Editor, Josh Hardman.
Matt Zorn, a prominent cannabis and psychedelics-focused attorney who made a name for himself in suing the government, has now joined its ranks. In accepting a role as Deputy General Counsel in the Department of Health and Human Services’ (HHS) Immediate Office of the General Counsel (OGC), Zorn appears to have quietly assumed the unprecedented position of ‘psychedelics czar’ in the second Trump administration.
Zorn has been a key adversary to governments of both stripes on drug-related issues, challenging them on matters like allowing research on cannabis for veterans with PTSD, preventing tryptamines from being scheduled, and fighting for patient access to psilocybin under Right to Try laws.
Now, he’s going inside the tent, with what looks to be a mandate over psychedelics-related matters.
Here, we recount Zorn’s path from ‘thorn in the side’ of the federal government to insider, what we might expect from him in his new role, and how insiders are reacting to the news.
***
Thorn in the Side to Government Insider
Zorn is no stranger to confronting the U.S. government and its federal agencies on matters broadly relating to drugs.
He has worked with researchers like Sue Sisley and his colleague Shane Pennington to sue the DEA over its effective rejection of her marijuana for PTSD in veterans study, with Psychedelic Alpha Editor-at-Large Graham Pechenik to lead a successful challenge against DEA’s plan to schedule five tryptamines, and held that same agency to task over other matters like access to psilocybin via Right to Try statute and its flouting of FOIA requests.
That FOIA-related saga led to the release of a letter from former Assistant Secretary of the HHS Rachel Levine to former DEA head Anne Milgram that recommended placing marijuana in Schedule III. The letter had first been mentioned in an August 2023 Bloomberg article and, in a sign of just how closely Zorn has followed these rescheduling-related matters, he filed that FOI request the very same day the Bloomberg article was published.
What’s more, in a sign of his persistence, he sued HHS in November 2023 after it failed to produce the requested letter.
Despite being deeply involved in psychedelics and cannabis legal issues, Zorn rejects the label of “psychedelic lawyer.” Being an effective lawyer is more important than identifying with any particular brand of lawyer, he has said.
And, whatever one thinks of Zorn, it is hard to argue that he has not been effective, including in securing an influential position in government.
***
A Psychedelics Czar
Zorn now serves as one of a half-dozen or so Deputy General Counsels at HHS’s OGC. While the office is technically tasked with providing legal advice across the agency, it has historically been instrumental in defining, defending, and implementing federal health policy, including, for example, the rollout of the Affordable Care Act.
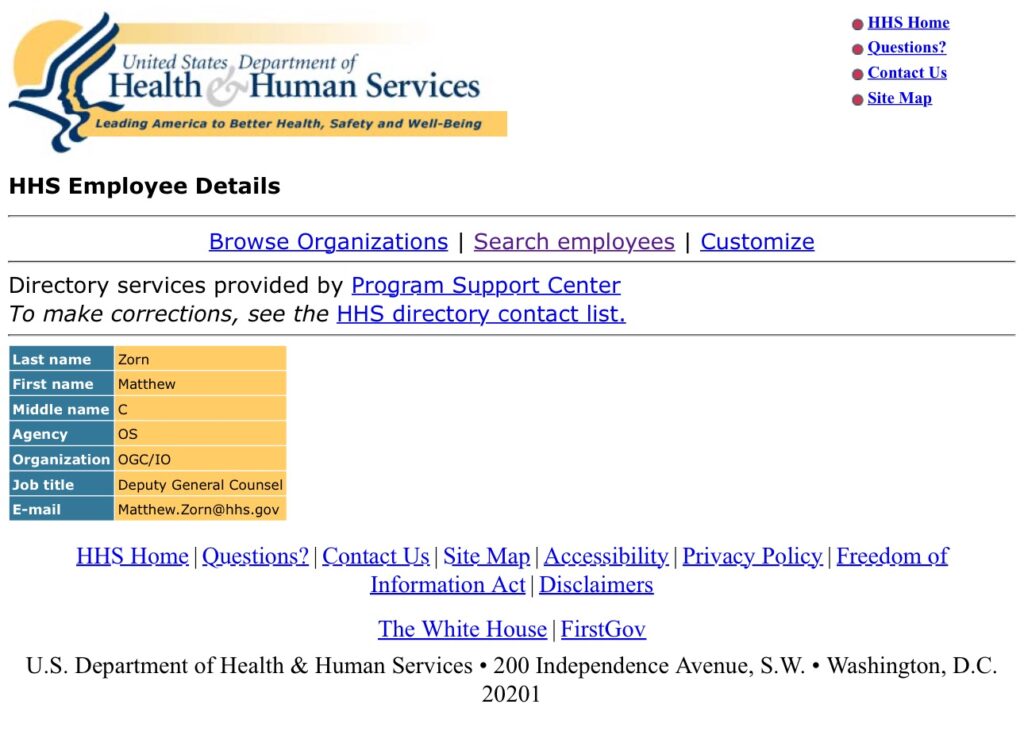
There are presently six Deputy General Counsel, according to the OGC’s website. Zorn is not yet represented there, but is reflected in an HHS staff directory.
Some of Zorn’s fellow counsel have clear areas of expertise and interest. Rujul Desai, for example, was appointed to his role last month and has a focus on matters relating to drug pricing and reimbursement. Eric Osterhues, meanwhile, played a prominent role in the Select Subcommittee on the Coronavirus Pandemic’s investigation of the origins of the virus, before being appointed to his Deputy General Counsel role at the OGC in March.
While the role of the OGC’s staff might seem quite vanilla, in that it is generally tasked with providing legal advice to HHS, they can wield meaningful influence over the broad portfolio of agencies and institutes under the Department. In the past, Deputy General Counsels have played important roles in flagship HHS programs, such as the Affordable Care Act, for example.
What’s more, its power has been centralised under the Trump administration. In March, the Department announced a reorganisation of the OGC, consolidating many of its regional offices (from ten to four) and creating a new position, the Chief Counsel for Food, Research and Drugs (which is presently occupied by Robert Foster after Hilary Perkins resigned after just two days on the job).
That consolidation echoes similar moves in the first Trump administration, according to Sidley Austin LLP, which “could potentially signal an effort to consolidate and expand HHS-OGC’s authority”.
What’s more, the Trump administration has made it clear that it is not particularly interested in the conventional remit of lawyers, so there is no reason to believe Zorn’s role will be limited to poring over minutiae.
Moreover, Zorn’s role—which had been rumoured for months—has been described by those close to the matter as a ‘psychedelics czar’, suggesting that his role will focus substantially on the matter.
***
“Bullish on Chaos”: Zorn’s Vision
Zorn has largely kept shtum, at least publicly, this year. But in a December 2024 interview with Psychedelic Alpha Editor Josh Hardman he offered a rare, unfiltered look at his thinking (and, perhaps, his unofficial CV for the role he now holds). (See Bullish on Chaos: Matt Zorn on How Psychedelics Could Benefit from Trump’s Second Term.)
In that discussion, Zorn expressed his excitement around a potential opening that the incoming Trump administration presented, in his opinion.
He expressed deep and characteristic frustrations with how the U.S. system of drug and medical regulation operates, and said he was excited that “someone like RFK Jr.” might be willing to ask ‘deeper questions’ about that system. “I do think that having someone who’s a bit of a chaos agent”, he said, “who is willing to actually peel back the layers and start asking these deeper, more fundamental questions can only be a good thing”…
To continue reading, please log in or join Pα+…
Join Pα+ Today
Independent data-driven reporting, analysis and commentary on the psychedelics space: from business and drug development through to policy reform and culture.
Already a member? Log In
✓ Regular Bulletins covering key topics and trends in the psychedelics space
✓ Regular articles and deep dives across psychedelic research, policy and business
✓ Interviews with insiders
✓ Monthly interactive database and commentary on psychedelic patents
✓ Quick-take analysis of major developments
✓ A Library of primers and explainers
✓ Access to our full back catalogue