Psychedelic Research Methods

Characterising and understanding the psychedelic experience, which is often deemed to be entirely ineffable, is no small feat. Some psychedelic researchers are employing neuroimaging techniques like PET, fMRI and fNIRS to take on this challenge. Their results might further our understanding of the underlying mechanisms through which psychedelics exert their apparent therapeutic effects, as well as informing clinical practice surrounding the delivery of psychedelic therapies.
In this panel, we spoke to two psychedelics researchers about their thoughts on the current state of neuroimaging in the field, as well as fruitful avenues for future studies and applications.
Part of our Year in Review series
Meet the Panelists

Parker Singleton
Parker Singleton is a PhD candidate in computational biology at Cornell University. His research characterizes and models the impact of psychedelic drugs on human brain dynamics.

Parker Singleton
Parker Singleton is a PhD candidate in computational biology at Cornell University. His research characterizes and models the impact of psychedelic drugs on human brain dynamics.

Drummond McCulloch
Drummond McCulloch has a background in drug design and pharmacology, and now works as a PhD Fellow at the Neurobiology Research Unit, Copenhagen, where he is supervised by Professors Patrick Fisher and Gitte Moos Knudsen. There, McCulloch coordinates clinical trials using fMRI, EEG and PET to evaluate the acute and persisting effects of psilocybin and LSD on the brain. “I am passionate about doing science better”, he told us.

Drummond McCulloch
Drummond McCulloch has a background in drug design and pharmacology, and now works as a PhD Fellow at the Neurobiology Research Unit, Copenhagen, where he is supervised by Professors Patrick Fisher and Gitte Moos Knudsen. There, McCulloch coordinates clinical trials using fMRI, EEG and PET to evaluate the acute and persisting effects of psilocybin and LSD on the brain. “I am passionate about doing science better”, he told us.
Researchers at UCL have announced plans to collaborate with a retreat provider in Mexico to conduct an EEG brain imaging study. What’s your opinion on the translatability of findings in a laboratory setting vs. more naturalistic settings?
Parker Singleton: I think naturalistic studies are both interesting and important. The key advantage of the ‘laboratory’ setting is the ability to somewhat recreate those same conditions anywhere in the world, which is important for replicability and comparison across studies. However, there’s nothing very natural about tripping inside of a giant whirring magnet, so it is a fairly niche set of conditions to study the brain under. Naturalistic observation may also teach us some new things that we otherwise would have missed, which we can then take back to the lab and study under more controlled conditions.
Drummond McCulloch: The answer in-part depends on the neuroimaging method at hand. We’ll be doing some acute EEG recordings in our upcoming 120-person study, where participants will be in a cosy, dimly lit room with two sitters and a curated playlist coming through top-end speakers, I think this will be somewhat analogous to a typical retreat, but not to something like a Gabonese iboga retreat where you get buried alive, dance for twenty-four hours, [and so on]. On the other hand, we have done psilocybin sessions in the MRI scanning environment which, although we tried to make it comfortable, is very dissimilar to a naturalistic setting. Ultimately, I am interested in the effects of psychedelics on the brain, and other stimuli will add noise to that system. If you’re interested in the effects of a psychedelic retreat on the brain, then of course, that noise becomes signal.
An individual’s response to a psychedelic is a little bit of a black box, right now, with plenty of inter-individual variability. Might neuroimaging help us in characterising these differences and predicting responses to psychedelics?
Parker Singleton: Yes, I do think so. We have seen a few instances now where baseline functional connectivity and serotonin 2a receptor availability mediate acute effects or post-acute cognitive change after psilocybin therapy (Preller, 2020; Doss, 2021; and Stenbæk, 2021). I think we will start to see more attempts at understanding inter-individual heterogeneity and predicting response to psychedelic compounds. Flora Moujaes of the Zurich group just published a very nice review discussing this from a computational psychiatry perspective. I’d also like to note here just how fortunate we are to have these compounds and to study their effects. I think we’ll see advances in computational psychiatry learned in studying psychedelics that can be easily applied to studying the brain under other conditions.
Drummond McCulloch: I think we’ll get a lot more bang for our buck in terms of prediction of response through just speaking to people and asking about their life, their personality and current state. That’s also a lot cheaper and less contraindicated. That’s not to say that we won’t try with neuroimaging, if we can show a replicable, predictive biomarker of treatment effect or acute effects then that would be extremely informative to the world of affective neuroscience. From a neuroscience perspective, I see psychedelics as a fantastic tool for understanding the brain activity patterns associated with mood and addictive disorders, perception, cognition and other functions, but I don’t envision patients getting fMRI scans before therapy any time soon.
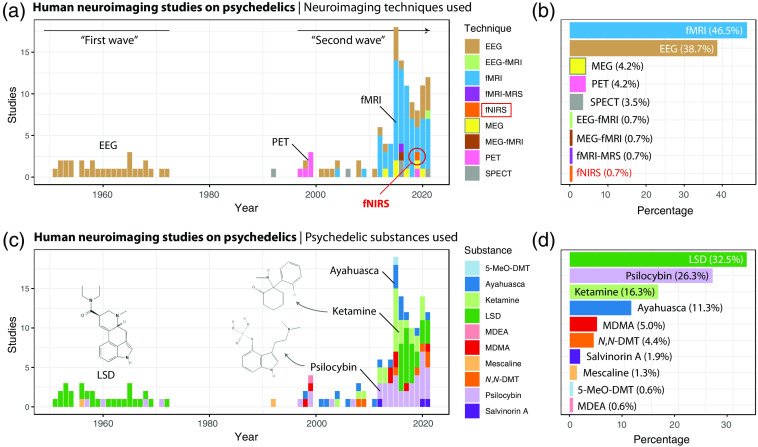
Mid-century psychedelic neuroimaging studies in humans were dominated by the use of EEG, with LSD being the most common drug used. Today, fMRI studies of psilocybin, ketamine and LSD are the favourites (Scholkmann and Vollenweider, 2023). What neuroimaging technology are you most excited about? And, is there a particular drug that you think is most propitious to neuroimaging?
Parker Singleton: The above referenced outlook on using fNIRS in psychedelics is definitely something I’m looking forward to. More broadly, I’m seeing more and more groups collecting multi-modal data (simultaneous EEG-fMRI for example) which really diversifies the kinds of questions we can ask by combining advantages across techniques. As far as favorite drugs go for neuroimaging, I’m a big fan of DMT. The things we can do with DMT from a pharmacokinetic standpoint are really exciting to me, as someone who is interested in modeling the brain effects of psychedelic compounds.
Drummond McCulloch: There is a “enhanced neuroplasticity” hypothesis of psychedelic therapeutic action that is quite popular. The most powerful tool we have at the moment for testing this hypothesis is the PET radiotracer UCB-J which binds to the synaptic protein SV2A and provides an estimate of synaptic density, a proxy for neuroplasticity. I am excited to see whether this increases or decreases following psychedelic administration. However, the mood changes seen in these trials is so rapid that I believe it quite unlikely that the changes in synaptic density are responsible, as these can take days to weeks to fully emerge, though they may be involved in the persistent nature of effects. We know that functional brain activity is rapidly disrupted following psychedelic intake, so this represents a more obvious starting point for investigating the changes associated with changes in wellbeing. Thus, neuroimaging methods like fMRI, EEG and MEG show a lot of promise here. PET occupancy studies are also essential for informing dosing strategies.
There’s some research coming out of Basel and Maastricht that suggest that drugs psychonauts consider different, like LSD, Psilocybin, Mescaline and 2CB are really quite hard to tell apart in a blinded administration setting, except for the differences in duration. If I was being pessimistic I’d say that at the end of the day these drugs are so alike, that any attempt to distinguish them using as noisy a tool as functional neuroimaging is a lost cause. To be more optimistic, perhaps functional neuroimaging will prove to be superior to subjective experience reporting in distinguishing the differences between these. As such, I’d encourage researchers to evaluate several of theses drugs if possible in the same scanner and individual so that we can have a shot at working these differences out. Regarding molecular neuroimaging, I think it’s critically important that we perform dose-occupancy studies with all of these drugs so we know whether any apparent differences are dose or drug related, so far this has only been done for psilocybin. Additionally, it will be very interesting to see if any of the new 2A agonists without psychedelic subjective effects, or combinations with antagonists, have similar effects on functional brain activity and whether the so-called psychoplastogens produce persisting increases in synaptic density (using UCB-J PET), and if this relates to changes in wellbeing.
Beyond research, can you envision a use for neuroimaging in the delivery of care surrounding psychedelic therapies and interventions?
Parker Singleton: Yes. Most of my research in the present is focused towards achieving that goal. I think EEG is the most accessible tool here, and I’m working towards building EEG models that will inform personalized decisions in psychedelic medicine. I believe other techniques will be useful to do this as well, however, more likely in special cases due to their higher costs. I’m really excited for the next generation of psychedelic neuroimaging studies that are coming down the pipeline. I can’t wait for us to learn more about the mechanisms of these compounds and how to more fully harness their effects for positive and enduring change.
Drummond McCulloch: There are three major outcomes of neuroimaging around psychedelics. Pre-imaging to discover the sorts of brains that respond well to psychedelic therapies, imaging during effects to discover the neural correlates of the psychedelic experience, and post-imaging to discover the changes in brain function and how these changes are associated with changes in wellbeing. None of these have immediate clinical application, but it would be short-sighted to disregard them because of this. The combination of neuroimaging and psychedelics presents an enormous opportunity to learn about mood and wellbeing in the brain. By isolating the essential therapeutic components of psychedelic action, new drugs and technologies can be developed to maximise efficacy and minimise risk. This is a long game, and we need to focus on developing a deeper understanding of the neurobiology underpinning these effects if we wish to ever develop better therapeutics.
Visit our Spotlight on Psychedelic Research Methods page for more perspectives on psychedelic research methods.
Part of our Year in Review series
This content is part of our 2022 Year in Review, which looks back at the past year through commentary and analysis, interviews and guest contributions.
Receive New Sections in Your Inbox
To receive future sections of the Review in your inbox, join our newsletter…