This Week:
- 💽 MindMed Collaborator’s Topline LSD for MDD Data
- 🪪 Oregon Issues Every Class of Psilocybin Licence, Except Service Centres
- 💂 UK Parliament to Discuss Psilocybin Rescheduling
- ⚖️ Psychedelic Policy Reform Updates
and lots more…
Psychedelic Sector News
MindMed Collaborator’s Topline LSD for MDD Data
Earlier this month, Matthias Liechti and Felix Mueller shared preliminary results from their Phase 2 LSD for major depressive disorder (MDD) trial.
The topline findings report “significant, rapid, durable and beneficial effects” of LSD
According to MindMed’s press release:
“The high dose lysergide regimen in which patients received 100 µg at their first dosing day and 200 µg at their second dosing day (separated by four weeks) resulted in statistically and clinically significant improvements on the primary endpoint, which was the change in clinician-rated Inventory of Depressive Symptomatology (IDS-C) scores 6 weeks after the first administration as compared to control (whether or not the patient received a second administration).”
As a reminder, MindMed collaborates with the Liechti Lab at University Hospital Basel, with exclusive access and rights to data generated by studies of lysergide and “other novel therapies”.
But, while this study is interesting and adds to our body of knowledge regarding the potential therapeutic effects of LSD, how useful is it to MindMed’s drug development pipeline?
Given that the company insists MM-120 is “MindMed’s proprietary drug candidate, a pharmacologically optimized form of LSD”, it’s going to have to produce clinical data demonstrating its safety and efficacy.
Indeed, that’s why it’s undertaking a Phase 2 trial of MM-120 for anxiety (NCT05407064) despite having acquired the rights to a Liechti Lab Phase 2 study that found LSD “produced long-lasting and notable reductions in anxiety and comorbid depression symptoms up to 16 weeks” (Holze et al., 2022).
Despite this fact, a MindMed representative told Psychedelic Alpha that the present Liechti Lab data, “does have bearing [on the MM-120 program] as it helps reinforce prior data on the potential of lysergide in anxiety and depression”.
We pushed MindMed on whether this LSD data could make it into a regulatory submission for MM-120. “It’s premature to comment on all of the [New Drug Application] requirements”, the representative told us, adding that the UHB studies contribute to “de-risking our pipeline, especially given the long history of clinical data for lysergide.”
Moving forward, an investor might like to see Liechti Lab focusing more closely on MindMed’s MM-120 program, which uses a different form of LSD and targets a different indication to the present data.
Side note: This tale also hints ever-so-slightly at the potential conflict between how drug developers seek to represent their products in different arenas. A drug developer might wish to tell their investors (and the patent office) that their drug is proprietary, while simultaneously seeking to leverage existing data of another drug when presenting to bodies like FDA.
Oregon Issues Every Class of Psilocybin Licence, Except Service Centres
According to data from Oregon Psilocybin Services (OPS), the division has now issued at least one of each licence type (as of April 24).
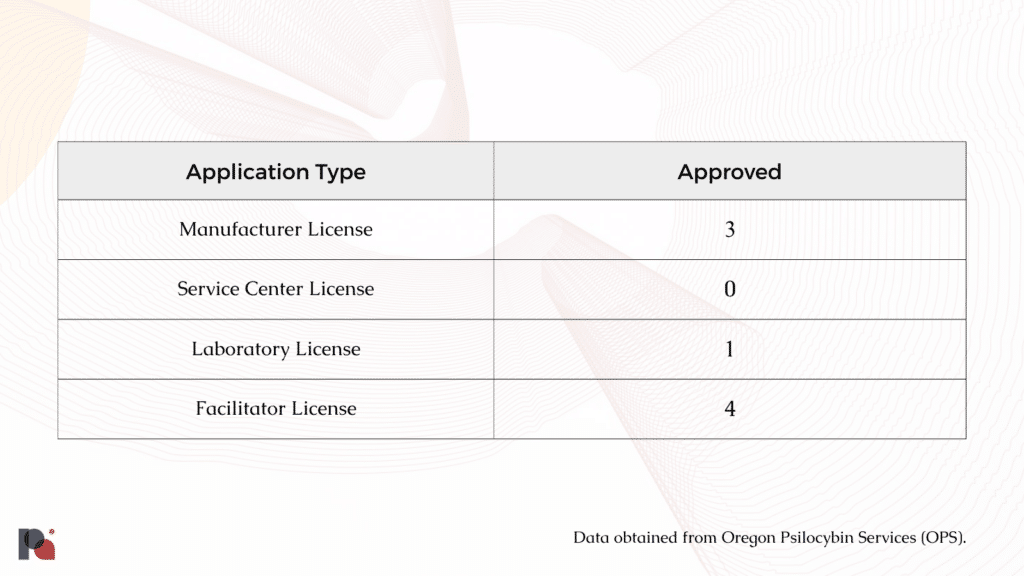
We also know that there are hundreds of ‘students’ on facilitator training programs. Having spent thousands of dollars for these programs, psilocybin facilitators are then faced with a $2,000 annual licensing fee.
Given that there are no licensed service centres at the time of writing, would-be facilitators might be wise to hold-off shelling out the $2,000 licence fee for the time being (the annual fee is agnostic as to whether the facilitator can, or does, practise).
This significant imbalance between facilitators and service centre applicants will likely mean there will be very few opportunities to provide legal psilocybin facilitation in the state for some time. It could be a long time before facilitators recoup the costs (both direct and opportunity costs) of their training and licence fees.
This lack of service centres, and associated costs, is further stoking Oregon’s underground mushroom market, according to the latest instalment from Anthony Effinger for Willamette Week.
In his reporting, Effinger speaks to Erica Zelfand, a psychedelic advocate and lead educator of InnerTrek’s psilocybin facilitator training program. “What’s the incentive for somebody to actually want to do this work above ground when they can go underground and make it much more accessible?”, she pondered.
A closer look at OPS licence application data suggests an imbalance at the level of manufacturers, too, with more manufacturers submitting applications (20) than service centres (17).
Our very own Josh Hardman covered considerations and challenges pertaining to Oregon’s non-medical legal psilocybin model, as well as a broader look at Colorado’s future model and other policy reforms, in reMind media’s latest Special Report.
UK Parliament to Discuss Psilocybin Rescheduling
According to the Conservative Drug Policy Reform Group (CDPRG), a debate on ‘Psilocybin Access Rights’ will be heard in the House of Commons in mid-May.
The debate will provide an opportunity for members of parliament (MPs) to make the case for better access to psilocybin researchers and ultimately patients, according to a communication from the group.
The applicants for the debate represent a variety of parties across the political spectrum, from the incumbent Conservatives through to the Scottish Nationalist Party and the main opposition party, Labour. More than two-dozen MPs supported the debate.
The debate is expected to take place on Thursday 18th May at 2pm, with up to three hours allocated. However, this is subject to the confirmation of Parliamentary business. We will provide updates in due course.
A couple of weeks ago, our Editor Josh Hardman took part in a panel discussion hosted by CDPRG, which has been campaigning for psilocybin rescheduling and the hearing of related debates in Parliament.
Featured Psychedelic Jobs
- Psychedelic Assisted Therapist (3mo residency in Cancun) with Beond.
- Executive Director of the American Psychedelic Practitioners Association.
Browse more roles and get more job posts to your inbox by signing up for alerts here. Make an account to join our free talent pool, too.
Miscellaneous News
- Rick Doblin leaves role as Executive Director of MAPS, becomes President. This isn’t the zinger that some commentators thought it to be. The MAPS Board of Directors appointed Doblin to the role of President, to whom the Executive Director (formerly Doblin, now former Deputy Director Kris Lotlikar) reports. Doblin told Psychedelic Alpha that he continues to serve as chief executive of MAPS”, and that both Lotlikar and himself report to the Board.
- University of Exeter launches psychedelics postgraduate certificate (but it’s not the first). During Breaking Convention, which was hosted at University of Exeter’s Streatham campus, news emerged that the University will launch a new programme: Psychedelics: Mind, Medicine, and Culture. More information about the postgraduate certificate (PGCert) is available here. It’s worth noting that, despite the university’s press release and other reporting, this isn’t the first time a university has launched a postgraduate certificate in psychedelics. University of Wisconsin offers a Capstone Certificate in Psychoactive Pharmaceutical Investigation, a “rapid, non-degree alternative” to its Master of Science program on the same topic. (Our editor, Josh Hardman, is presenting on the psychedelic ‘industry’ at a forthcoming lecture for one of the courses in this certificate program.)
- Blinding isn’t just a psychedelic science issue? As mentioned by Sandeep Nayak on Twitter, a recent article in BMJ Mental Health assessed blinding success in trials of antipsychotics for acute treatment of schizophrenia. Of the four studies that undertook blinding assessments, blinding was broken in all of them with the proportion of correctly guessed treatment allocation raging between 70% and 91%. Two takeaways here: 1) blinding assessments are incredibly rare in the observed trials, with fewer than 2% of studies employing them; 2) when blinding is assessed in these trials, it suggests it is broken in the vast majority of cases. As Balázs Szigeti said on Twitter: “the lack of blinding assessment, and that when it is assessed it’s often found to be broken, is not just a psychedelic science issue, it’s more widespread”.
- “MDMA expected to be approved to treat PTSD by October”, reads a New York Post headline. While MAPS PBC hopes to submit an NDA by then, it will almost certainly not receive a decision from FDA until 2024.
Psychedelic Policy Reform Updates
This week, we’re providing a look ‘under the hood’ at how we track psychedelic drug policy reform across the United States. The below updates are more granular than those in our Psychedelic Legalization & Decriminalization Tracker, which is produced with the support of experts at Calyx Law and Emerge Law Group.
These updates reflect the period April 10 – 28, 2023
SB 303 – Oregon
Relating to Psilocybin Services; Prescribing and Effective Date
Introduced: January 6, 2023
Latest Action Date: April 20, 2023
Latest Action: Referred to Behavioural Health and Health Care
Next Action: Work Session Schedule for May 8, 2023
On April 20, 2023, SB 303 was referred by the House to the Behavioural Health and Health Care committee. The bill will now be reviewed and the committee will hold a public hearing and work session which are scheduled for May 3rd and May 8th respectively. If passed into law, SB 303 would require psilocybin service centres and facilitators to collect and report data on client details, adverse events, and other related information.
SB 23-290 – Colorado
Natural Medicine Regulation and Legalization
Introduced: April 18, 2023
Latest Action Date: April 27, 2023
Latest Action: Referred to Appropriations
Next Action: To be assigned to committee by House
On April 18, 2023, SB 23-290 was introduced into the Colorado Senate. The bill, which passed through the Senate within a week, was introduced in the House on April 25, 2023. The amended bill has recently been referred to Appropriations by the House Committee on Finance and if referred favourably, will advance through subsequent readings in the House before it is ultimately voted on. If passed into law, SB 23-290 would establish the regulatory framework for the implementation of Colorado’s Natural Medicine Health Act which was passed in November 2022.
HB 1340 – Hawaii
Relating to Mental Health
Introduced: January 25, 2023
Latest Action Date: April 27, 2023
Latest Action: Conference Committee Meeting
Next Action: Conference Committee Meeting to Reconvene on April 28, 2023
On April 11, 2023, Hawaii’s HB 1340 was passed by the Senate with a total of 24 votes for and none against. However, on April 13, after the amended bill was returned from the Senate, the House issued a notice regarding its disagreements with Senate amendments. HB 1340 has been subsequently referred to a conference committee where areas of disagreement will be considered. If signed into law, HB 1340 would establish the “Beneficial Treatments Advisory Council” to, amongst other things, “develop a long-term strategic plan to ensure the availability of therapeutic psilocybin, psilocybin-based products, and methylenedioxymethamphetamine that are safe, accessible, and affordable…”
SB 58 – California
Controlled Substances: Decriminalization of Certain Hallucinogenic Substances
Introduced: December 16, 2022
Latest Action Date: April 25, 2023
Latest Action: Senate hearing set for May 1, 2023
Next Action: Senate hearing
On April 25, 2023, after being re-referred to the Committee on Appropriations, a hearing date was set for California’s SB 58. Following the hearing, the committee must vote on passing or defeating the bill before it can be advanced further in the legislative process. If enacted, SB 58 would, amongst other things, “make lawful the possession, preparation, obtaining, transfer, as specified, or transportation of, specified quantities of psilocybin, psilocyn, dimethyltryptamine (DMT) , ibogaine, and mescaline, for personal use or facilitated or supported use, as defined, by and with persons 21 years of age or older.”
SB 242 – Nevada
Requires the Department of Health and Human Services to establish the Psychedelic Medicines Working Group
Introduced: March 9, 2023
Latest Action Date: April 24, 2023
Latest Action: Re-referred to Committee on Finance
Next Action: Awaiting committee recommendation/determination
On April 21, 2023, the Committee on Health and Human Services recommended SB 242 pass as amended. However, the bill was subsequently re-referred to the Committed on Finance for further consideration. So long as the committee does not vote to postpone the consideration of SB 242, the bill will progress further through the legislative process. If enacted, the bill would require Nevada’s Department of Health and Human Services to “establish the Psychedelic Medicines Working Group to study certain issues relating to the therapeutic use of entheogens”.
SB 5263 – Washington
Concerning Access to Psilocybin Services by Individuals 21 Years of Age and Older
Introduced: January 11, 2023
Latest Action Date: April 20, 2023
Latest Action: Delivered to Governor
Next Action: Awaiting final action/inaction from Governor
On April 20, 2023, Washington’s SB 5263 was delivered to the state’s governor after being accepted by both the House and Senate. The bill will now foreseeably be signed into law by the governor. SB 5263, when introduced, sought to regulate psilocybin services for individuals over the age of 21 in the state. However, SB 5263 underwent some significant changes after a Senate committee approved a substitute bill which narrowed the bill’s scope in late February 2023. Consequently, SB 5263 is now focused on the establishment of “an advisory board, interagency work group, and task force to provide advice and recommendations” for regulating access to psilocybin services.
HB 727 – North Carolina
Breakthrough Therapies Research/Advisory Act
Introduced: April 18, 2023
Latest Action Date: April 19, 2023
Latest Action: Referred to Committee on Health
Next Action: Awaiting committee recommendation/determination
On April 18, 2023, HB 727 was introduced in North Carolina. After passing its first reading on April 19th, the bill was referred to the House Committee on Health. If referred favourably, the bill will pass through the committees on Appropriations and subsequently Rules, Calendar, and Operations before proceeding through the nearly steps early in the legislative process. If eventually signed into law, HB 727 would establish a Breakthrough Therapies Research Grant Fund that would provide two $2.5m grants to North Carolina research projects investigating the use of MDMA or Psilocybin to treat certain psychiatric indications in qualifying patient populations.
HB 6734 – Connecticut
An Act Concerning the Decriminalization of Possession of Small Amounts of Psilocybin
Introduced: February 22, 2023
Latest Action Date: April 17, 2023
Latest Action: Bill assigned House Calendar Number
Next Action: To be debated and voted on in the House
On April 17, 2023, after passing out of committee, HB 6734 was given a favourable report and was consequently tabled for the Calendar in the House. HB 6734 will now be debated and amendments to the bill can be proposed before a final House vote occurs. Should HB 6734 eventually be signed into law, criminal penalties for possession of up to one-half ounce of psilocybin would be eliminated.
HF 240 – Iowa
A Bill for An Act Removing Psilocybin and Psilocyn from the List of Substances Classified As Schedule I Controlled Substances Under Iowa’s Uniform Controlled Substances Act
Introduced: 8/2/2023 February 2, 2023
Latest Action Date: April 11, 2023
Latest Action: Subcommittee recommends passage
Next Action: Awaiting committee recommendation/determination
On April 11, 2023, an Iowa subcommittee, through a vote, recommended the passage of HF 240. The full committee will now discuss the subcommittee’s conclusions and present its recommendations to the House. If enacted, the bill would see psilocybin and psilocyn removed from Iowa’s list of schedule I controlled substances under its uniform controlled substances act.
Weekend Reading
- Nature: US could soon approve MDMA therapy — opening an era of psychedelic medicine
- TIME: The Latest Promising Long COVID Treatment? Psychedelic Drugs
- Washington Post: Executive behind ChatGPT pushes for a new revolution: Psychedelics
- WSJ: Book Review of Mike Jay’s ‘Psychonauts’
- ESPN: Pain, hope, science collide as athletes turn to magic mushrooms
- CBC: People were using psychedelic drugs in Bronze Age Europe, study finds
- New Scientist: Psychedelics may increase entropy in the brain’s vision centre
Special Report: The Opportunities and Challenges in Oregon, Colorado and Future Non-Medical Marketplaces
Weekly Bulletins
Join our newsletter to have our Weekly Bulletin delivered to your inbox every Friday evening. We summarise the week’s most important developments and share our Weekend Reading suggestions.



