This week’s bulletin (which is coming a few days later than our usual Friday communiqué) offers a deep dive into COMPASS Pathways’ topline Phase 2b results, which were released last Tuesday.
In this long-read piece we seek to begin contextualising the preliminary safety and efficacy data from this influential trial, and end by summarising the next steps for COMPASS.
Deconstructing COMPASS Pathways’ Phase 2b Results
Topline results from COMPASS Pathways’ Phase 2b trial of its proprietary psilocybin formulation, COMP360, for treatment-resistant depression (TRD) have caused quite a stir among researchers and investors alike since their publication last week.
Despite what appeared to be positive results, the company’s stock price took a tumble and dragged much of the sector down with it. Perhaps the most frequent question among investors is: why is the stock price falling? We won’t seek to address that in this piece, as attempting to apply rationality to markets is often a fool’s errand. Rather, we will focus more substantively on the preliminary results, to which many have responded with confusion.
Much of the confused reaction caused by these results most likely stems from a lack of adequate frames of reference or cases for comparison. For example, what should we compare or contrast these results to? Should we look toward other clinical or observational studies of psychedelics? Or, other treatments targeting the same population, in this case those suffering treatment-resistant depression?
In a similar vein, how should we contextualise the serious adverse events observed in the present study? Should we assess the background level of suicidal behaviour among this patient population, for example?
This article aims to begin providing such context, though it must be stressed that we are working with an incomplete set of results. That is, we only have the topline results, published by COMPASS Pathways, at present.
When further data is released in due course, you can be sure to see more in-depth reaction from experts across the space.
This Phase 2b trial is the largest randomized controlled double-blind trial of psilocybin in the world, enrolling 233 patients across North America and Europe.
COMPASS Pathways attempted to standardize their psilocybin-assisted therapy protocol as far as possible among these trial sites and individuals: a significant undertaking given the heterogeneity of psychedelic-assisted therapies.
Overcoming the incongruities between the idiosyncratic psychedelic-assisted therapy modality and the objectivity and standardization demanded by the clinical trial regime is certainly no small feat. In fact, it was a significant contributor to the downfall of psychedelic research in the first place (see Oram, 2014; Bonson, 2017; or, Hall, 2021 for a broader overview).
The population targeted by this psychedelic intervention is those with treatment-resistant depression (TRD). These individuals, of which there are thought to be at least 100 million worldwide, have failed to respond to at least two existing antidepressant treatments.
Participants were given a single dose of psilocybin from 3 dosing options (1mg, 10mg, or 25mg) alongside psychological support. Note that the 1mg dose is, for all intents and purposes, a placebo.
So, did it “work”?
Preliminary Evidence of Efficacy
Remember: Phase 2 studies assess the safety and efficacy of an intervention across a range of doses. Due to the relatively small numbers of patients involved, conclusions about overall efficacy cannot be drawn at this stage. These conclusions are made as a result of Phase 3 trials, with at least two Phase 3s usually required to provide sufficient evidence. See our Primer on the drug development process for more.
When evaluating a new treatment, like COMP360 in conjunction with psychological support, the two broad buckets of success pertain to safety and efficacy. First, we will review the preliminary evidence of efficacy provided by these topline results.
In this study, the primary endpoint was the Montgomery–Åsberg Depression Rating Scale (MADRS), a commonly-used clinician-rated scale which measures the severity of a participant’s depression.
The 25mg dose of COMP360 led to a 6.6 point reduction in MADRS at week 3 versus the 1mg dose, a clinically significant change (Turkoz et al., 2020) in depression severity. For context, according to COMPASS’ Chief Medical Officer Guy Goodwin, the average reduction in MADRS after 6 weeks of SSRI usage is somewhere between 2 and 3. In line with this claim, a meta-analysis by Kennedy et al. (2009) found that escitalopram, a leading SSRI, was associated with a 2.3 point reduction on the MADRS compared with placebo.
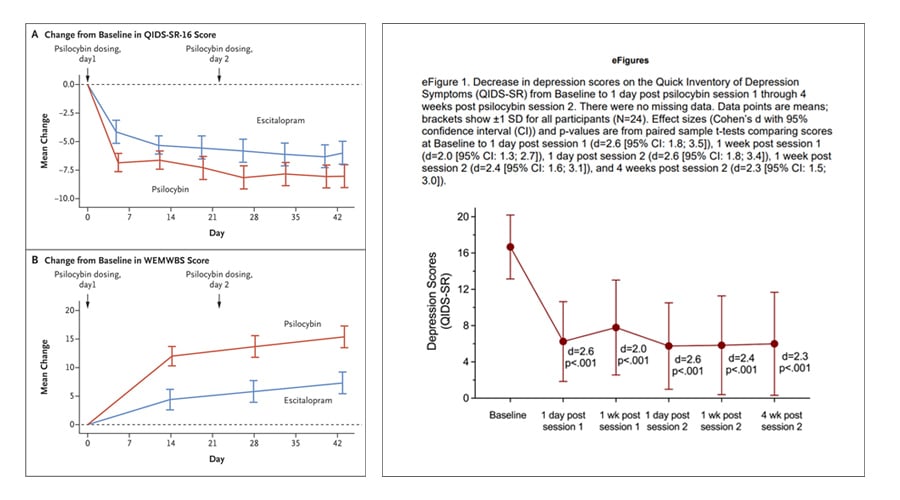
This effect is similar in magnitude to that seen in other studies of psilocybin for depressive disorders. Robin Carhart-Harris et al.’s (2021) study of psilocybin versus escitalopram for depression, for example, found a slightly larger change in MADRS of -7.2 at week 6 (note that MADRS were among the secondary outcomes for this study). The study, which was smaller at just 59 participants and targeted major depressive disorder (MDD), administered 2 doses 3 weeks apart.
***
In the present study nearly a quarter (24.1%) of patients in the 25mg group were sustained responders* at week 12, versus just over one-in-ten (10.1%) in the 1mg group.
* “Patients meeting the MADRS response criteria at week 3 and at week 12, and at least one visit out of week 6 and week 9, and who did not start any new treatments for depression”
Given that the participants in this trial have tried at least two existing antidepressant therapies to no avail, the fact that nearly a quarter of those in the 25mg group were sustained responders after three months is a significant finding, and a positive outcome.
However, it should be noted that 10.1% of the 1mg group were also sustained responders at the three-month mark. This was also observed in MAPS’ first Phase 3 trial of MDMA-assisted psychotherapy for PTSD (NCT03537014), in which patients in the placebo group similarly experienced significant reductions in PTSD (Mitchell et al., 2021).
The nature of the psychological support provided in COMPASS Pathways’ trial is not clear, so it is difficult to make assumptions around how much this may have contributed to improvements observed in some participants in the 1mg ‘placebo’ group. This improvement may be due to more generalisable effects of clinical trial participation, such as expectancy effects.
***
While the observed efficacy of COMP360 combined with psychological support is, by definition, remarkable in the context of the treatment-resistant depression population, it must be remembered that the majority of those in the 25mg group did not exhibit a sustained response.
This should serve as an important reminder that psilocybin-assisted therapy is by no means a panacea, but rather another potential tool in the pharmacotherapy toolkit.
***
Might more doses result in greater efficacy?
COMPASS’ protocol administers a single dose of psilocybin, while other studies have administered multiple.
For example, the aforementioned study of psilocybin versus escitalopram (Carhart-Harris et al., 2021) employed two doses, three weeks apart. Another study of psilocybin for MDD also administered 2 sessions of drug-assisted therapy (Davis et al., 2020).
This has led some to ask: might a second or third session of COMP360 increase response to the therapy, and/or its durability?
A cursory look at data from the aforementioned 2-session studies for MDD, however, imply that the second session may not have a significant additive effect in terms of reducing depression severity.
Speed: how fast-acting is COMP360?
When we talk about efficacy, speed of onset is also of relevance. Antidepressants like SSRIs, for example, are frequently lamented for their slow onset time with many patients failing to feel benefits for up to six weeks, if at all.
Ketamine, on the other hand, has a very rapid onset and frequently provides antidepressant effects within hours of administration (Duman, 2018). This makes it useful in the provision of an acute antidepressant intervention, such as in cases where patients present with suicidal ideation and would benefit from rapid relief.
The topline results from this COMP360 trial reiterate that psilocybin-assisted therapy has a rapid onset of action, with therapeutic benefit observable at day 2 (see below). However, it should be remembered that psilocybin is administered only after a course of preparatory psychotherapy.
Durability: how long do the effects last?
As you can see from the chart above, COMP360 appears durable at week 12*, with 24% of patients in the 25mg group being sustained responders vs. 10% in the 1mg cohort. This is significant when compared ketamine, for example, which sustains an antidepressant response for a much shorter period of time: often in the region of one week (Matveychuk et al., 2020).
* It should be noted that the statistical significance of this durability is uncertain, due to the manner in which COMPASS analysed its results (see this Tweet from Michael Haichin for more).
Due to the nature of the study design, it is not clear how durable the effects of the company’s psilocybin-assisted therapy might be beyond three months. Data on this topic will be of key interest to payers, and may be augmented by a long-term follow-up study (NCT04519957) of participants in this Phase 2b trial and one other COMPASS study.
Nonetheless, this preliminary data on durability is promising and outstrips that of ketamine, for example.
Is it safe?
Safety and tolerability in the Phase 2b trial
While the vast majority of side effects of COMP360 were mild to moderate in nature (such as headaches or nausea), the existence of serious adverse events among a number of patients has drawn a great deal of attention.
12 patients in the study reported treatment-emergent serious adverse events (TESAEs), with 5 of those dozen in the 25mg group and 6 in the 10mg group. These TESAEs generally revolved around suicidal ideation and behaviour and self-injury. In contrast, only one patient in the 1mg group reported a serious adverse event.
Speaking to STAT, COMPASS co-founder and president Lars Christian Wilde suggested that these numbers are small enough for the difference between the treatment arms to lack statistical significance.
Even still, the correlation is hard to ignore. This led many observers to question the safety profile of COMP360, given the apparent connection between an ‘active’ dose of COMP360 and serious adverse events.
COMPASS Pathways attempted to address such observations in an email to New Atlas, explaining:
“This assumes a causal relationship between the drug and all the reported adverse reactions – we don’t know that to be the case. COMP360 was administered on a single day, on which there were mild/moderate adverse reactions for some patients (e.g., headache, nausea, fatigue). However, of the 12 patients experiencing serious adverse events, only 1 of them occurred within 24 hours of the dosing. The other 11 experienced theirs up to 62 days later. Therefore it is not clear that there is a direct link with administration of the drug.”
Furthermore, CMO Guy Goodwin explained on Tuesday’s investor call that those who exhibited suicidal behaviour did so over a month post-treatment, though there was at least one case of suicidal ideation early on in the trial.
Goodwin, along with the company’s CEO George Goldsmith, were also keen to emphasize that suicidal behaviour was observed primarily among those who failed to respond to the therapy, ‘non-responders’.
***
Are inflated expectations leading to despair among non-responders?
While qualitative details of the circumstances leading up to and surrounding this suicidal behaviour are lacking at present, might we expect that the non-responders were feeling especially hopeless after failing to respond to COMP360?
The combination of a great deal of hype around the potential (or, ‘promise’) of psychedelic-assisted therapy, plus the obviousness of being ‘on’ a 25mg dose of psilocybin, could have led to a feeling of despair and hopelessness among those in receipt of COMP360 who did not feel that their TRD had been addressed.
This exuberance and hype isn’t reserved for COMPASS’ proprietary psilocybin formulation: it’s present across psychedelic-assisted therapies.
Speaking to Salon, a participant in MAPS’ Phase 3 MDMA-for-PTSD trials touched on these great expectations, saying:
“Everything that I read, everything that I heard about, was how this stuff will fix you. This stuff is going to make you better. It’s going to make you unbroken. It’s going to put all the pieces back together.”
Tehseen Noorani has coined the “Pollan Effect” to refer to these inflated expectations of psychedelic-assisted therapy in a nod to Michael Pollan’s best-selling book on the topic, How to Change Your Mind. Speaking to Noorani, Johns Hopkins psychedelics researcher Albert Garcia-Romeu explained that this phenomenon is “a huge problem,” with participants coming to trials with “all these preconceived ideas.”
Might these inflated expectations of, and hopes for, psychedelic-assisted therapy among trial participants be generating outsized despair and hopelessness among non-responders?
If so, what can be done about these outsized expectations of psychedelic-assisted therapies? Or, if managing expectations is implausible given the public saliency of psychedelics at present, should we be aiming to manage the inevitable disappointment and attendant suicidality among non-responders?
In the therapy protocol employed by this Phase 2b trial, participants have preparatory sessions with therapists, in-session support, and post-session integration. Yet, this failed to prevent suicidal behaviours.
If the management of suicidal ideation and behaviours among non-responders has proven difficult in this closely-managed protocol, what might we expect in much less regulated contexts, such as in localities that are decriminalizing psychedelics? It’s certainly something worth watching closely.
***
Other responses to the incidence of serious adverse events in the trial were presented, including perhaps the most foundational one that reminds us that suicidal ideation and behaviour is prevalent in this patient population, and as such is to be expected in such trials.
COMPASS highlighted this fact in its press release, stating:
“These TESAEs included suicidal behaviour, intentional self-injury, and suicidal ideation, which are regularly observed in a treatment-resistant depression patient population.”
Researcher James Rucker forwarded a similar reasoning explaining, “this is a TRD population hence morbidity is high anyway.”
***
Comparing these SAEs to other studies of psychedelic-assisted therapies
There were 0 SAEs in Carhart-Harris et al.’s psilocybin for depression study (2021), though participants were MDD sufferers as opposed to TRD, and as such may not have equivalent levels of desperation and thus despair when unresponsive.
When compared to Spravato, Janssen’s esketamine nasal spray which gained approval for TRD, the incidence of SAEs in the COMP360 trial appear much more common. While there were three completed suicides throughout the esketamine trials, a review of the New Drug Application (NDA) for the drug’s use in TRD reveals far fewer SAEs:
As you can see, SAEs were more commonly reported in the esketamine group compared to the placebo, but at a much lower incidence than in the COMP360 Phase 2b which were reported as follows:
- 6.3% (5 patients) in 25mg group
- 8.0% (6 patients) in 10 mg group
- 1.3% (1 patient) in 1mg group
In MAPS’ first Phase 3 of MDMA-assisted therapy for PTSD, meanwhile, there was no observed increase in adverse events related to suicidality in the MDMA group. Rather, in the placebo group there was 1 SAE of suicidal behaviour during the trial, and another SAE of suicidal ideation leading to self-hospitalization.
There were also more adverse events of special interest (AESIs; suicidal ideation, suicidal behaviour, self-harm in the context of suicidal ideation) in the placebo group during the MAPS study, with 5 in the placebo group and 3 in the MDMA cohort. Serious suicidal ideation was minimal during the study and occurred almost entirely in the placebo arm.
***
It must be noted that proper analysis of the SAEs observed in this trial of COMP360 is difficult at present, as only topline results have been made available.
One thing is for certain: further analysis and full publication of the data will allow for a closer understanding of these serious adverse events.
What’s Next?
COMPASS Prepares for Phase 3
COMPASS will meet with the FDA in early 2022 to discuss its Phase 3 design, and will hope to begin the trial by Q3. It is almost certain that the company will employ the 25mg dose in the study.
In the coming weeks and months, the company’s team will continue poring over qualitative and quantitative data from the trial in an attempt to optimize its therapy. Speaking to investors on Tuesday’s call, CEO George Goldsmith said,
“We have machine learning now looking at transcripts across the trial participants, really starting to understand a deeper level of patterns, including changes to patient narrative, therapist actions that are then correlated with particular outcomes. This is unprecedented data, and we’ll be digging deep into that to look at how we can in fact, optimize therapy.”
Might there be some characteristics that identify those likely to respond? If the company is able to identify those patients likely to respond positively and in a clinically significant magnitude prior to administering the therapy, this could have important implications for both cost and safety.
The company will also be keen to gain a more accurate picture of the relationship between its treatment and suicidal ideation and behaviours. On the same investor call, CMO Guy Goodwin said,
“Our plan is to look at [the cases] in as much detail as we can, but in addition, in the secondary analyses, to look at the Columbia suicide scales that were collected on every patient at every visit. That will give us, I think, a much more accurate understanding of the relationship between treatment and both suicidal ideation and suicidal action.”
The company also has a number of smaller trials underway, including an open-label trial that evaluates whether patients might be able to continue taking SSRIs during the psilocybin-assisted therapy. The findings of such studies may inform the design of the company’s Phase 3 trials.
The Verdict
According to the preliminary evidence offered by this Phase 2b trial, COMP360 delivered alongside psychological support is fast-acting and effective in a not-insignificant portion of TRD sufferers. It also appears to be durable up to the 12 week endpoint, which is favourable when compared to other novel treatments such as ketamine.
Given that this population is—by definition—in desperate need of novel therapies to address their depression, these preliminary results may outweigh safety concerns surrounding the treatment. While certainly warranting further investigation, these safety concerns must also be understood relative to the baseline levels of suicidal ideation and behaviour within this population, as well as the safety profiles of alternative treatments.
While addressing treatment-resistant depression in around a quarter of recipients of high-dose COMP360 is remarkable, the inverse statistic is that a majority of participants in the 25mg group did not enter remission and continue to suffer a TRD diagnosis. This should serve as a sobering reminder that psilocybin-assisted therapies are by no means a panacea.
Other Company News
- COMPASS Pathways announces financial results and business highlights for Q3 2021;
- Cybin announces positive CYB003 data demonstrating advantages over oral psilocybin;
- MYND executes LOI to enter into clinical research collaboration with Revitalist;
- Nova Mentis files genetic neuroinflammatory disease patent;
- Numinus to host extension of MAPS-sponsored MDMA-assisted therapy for PTSD trials;
- PharmaTher announces positive research results for psilocybin microneedle patch;
- Silo Pharma reports Q3 results and operating highlights.
Read more on our News page.
Week(end) Reading
Due to the length of the above deep dive, and the fact that this is being published on a Monday, we’re sharing just a few reading suggestions this week…
- The Times: Magic mushrooms show some success in battle against depression
- Rolling Stone: U.S. Government Funds First Therapeutic Psilocybin Research in 50 Years
- Nature Translational Psychiatry: Psilocybin therapy increases cognitive and neural flexibility in patients with major depressive disorder
- The Guardian: Will the magic of psychedelics transform psychiatry?
- The New York Times: Veterans Have Become Unlikely Lobbyists in Push to Legalize Psychedelic Drugs
Weekly Bulletins
Join our newsletter to have our Weekly Bulletin delivered to your inbox every Friday evening. We summarise the week’s most important developments and share our Weekend Reading suggestions.
Live Updates
Join us on Twitter for the latest news and analysis.
Other Channels
You can also find us on LinkedIn, Instragram, and Facebook.