This week has been characterised by a number of developments on the legal and regulatory side, some of which we attempt to unpack below. These include the issuing of an Opinion on one of Compass Pathways’ UK patents; efforts to reform psilocybin regulations in Canada; and, the continued denial of data protection for Janssen’s Spravato product.
Given the length of the news section this week, the weekend reading section features just one documentary today.
Psychedelic Sector News
UK Patent Examiner Issues Negative Opinion on Compass Pathways Patent
A patent examiner at the UK IP Office has issued an opinion on Compass Pathways’ UK Polymorph A patent (GB2572023), having received a request for opinion from Kohn & Associates acting on behalf of Freedom to Operate. The examiner found a number of claims to be lacking an “inventive step,” a foundational tenet of the patent regime.
While the opinion was issued in late July, Shayla Love brought the document to attention after the VICE journalist shared a thread on Twitter earlier this week.
Of interest to those following Compass Pathways' patents on psilocybin: On July 28 an examiner at the UK IP Office issued an opinion on Compass' UK patent: "I consider that claims 1, 3 and 10-20 are not inventive, based on Folen and Nichols." 1/ https://t.co/lLyB7WcS8w pic.twitter.com/xj1oZP1k4P
— Shayla Love (@shayla__love) August 2, 2021
Crucially, the opinion considers claims 1, 3, and 10 through 20, to be “not inventive,” due to their similarity to work found in publications by Folen and Nichols.
We reached out to Compass for comment, and a representative told us the following:
“We have received a non-binding opinion from the UK IP Office, questioning aspects of some of the claims in one of our granted UK patents. The opinion came in response to a “Request for opinion” from a third party and is just that: a non-binding opinion. The opinion does not invalidate our patent or any of its claims.
We remain confident in the strength and defensibility of our patents.”
However, in a piece published yesterday, Psymposia—”a non-profit media organization that offers leftist perspectives on drugs, politics, and culture”—outlined the Opinion and obtained comment from Dr. David Nichols, the respected psychedelics researcher and named co-inventor on the patent. According to Psymposia, Nichols “believes [the patent examiner’s] opinion could be accurate.”
“The opinion is very comprehensive,” Nichols said. “I am not a patent attorney, so I cannot opine on whether it is precisely correct. I assume that examiner Bellia is well versed on patents and that his opinion is a valid one.”
Also speaking to Psymposia, Psilocybin Alpha Editor-at-Large Graham Pechenik explained the significance of the Opinion:
Pechenik said that in layman’s terms, Bellia’s conclusion “means that the UK patent examiner believes that those claims are not inventive, and should be invalid, but the opinion is not binding and does not have any immediate effect on Compass’ patent, although it could subsequently be revoked.”
The full Opinion can be accessed here.
Buoyed By Positive Opinion Polls, Canadian Non-Profits Push for Psilocybin Policy Reforms
A new poll, commissioned by the Canadian Psychedelic Association, has highlighted widespread support for legal access to psilocybin-assisted therapy among Canadians. One of the headline findings is that 82% of Canadians approve the use of psilocybin-assisted therapy for those suffering from an end-of-life illness, a landslide opinion.
Seeking to capitalize on an apparently propitious public mood, the CPA announced a ‘Memorandum of Regulatory Approval’ (MORA), the product of 10 months of discussion with a variety of stakeholders. Cory Firth, Executive Director of the CPA, explained:
“The MORA was prepared by some of the best researchers, industry, legal and regulatory experts in Canada. As the voice of psychedelics in Canada we made sure that no stone was left unturned in our efforts to bring timely and effective regulatory change to Canadians at end-of-life and suffering from various treatment-resistant mental health conditions.”
Field Trip’s Ronan Levy, a CPA member, described the MORA as forwarding a “well-considered, balanced approach,” adding that “cost-benefit analysis strongly favours prompt access to psychedelic therapies.”
Victoria-based TheraPsil, which has helped over 50 Canadians receive section 56 exemptions to access psilocybin-assisted therapy to date, is also pressing the Minister of Health for more substantive regulatory changes. Section 56 exemptions are issued at the Minister’s discretion, but TheraPsil believes that a patient’s healthcare provider is better placed to regulate access to psilocybin for medical purposes.
To this end, on Wednesday the non-profit published a first draft of proposed ‘Access to Psilocybin for Medical Purposes Regulations,’ or ‘APMPR.’ The proposed regulations are somewhat similar to those that govern cannabis at the federal level, regulating not only access to psilocybin, but also the manufacture and labelling of the product itself. TheraPsil believes this will “provide for greater safety and consistency in quality-controlled psilocybin material,” as opposed to the current situation where even those granted exemptions are forced to acquire psilocybin from unregulated sources.
Having conducted their own poll earlier this summer, the group also expects public support in their efforts.
No Data Protection in Canada for Ketamine’s Chemical Cousin, Esketamine (Spravato)
Canada’s Federal Court of Appeals (FCA) has upheld the Minister of Health’s refusal to grant data protection for Janssen’s Spravato (esketamine) product.
Spravato, a nasal spray formulation of esketamine indicated for major depressive disorder (MDD), was approved by the FDA in 2019, with Health Canada following suit in 2020. In the U.S., it was approved by a 14-2 vote and was lauded as the first drug in 35 years that appeared to fight depression via a different approach to conventional antidepressants.
The product is fast-acting, often providing relief from depressive symptoms within a few hours, as opposed to the weeks it can take for many conventional antidepressants to take effect. As such, it is especially useful in acute and severe cases of MDD, such as those accompanied by suicidal ideation.
Importantly, esketamine hydrochloride is an enantiomer of ketamine hydrochloride. An enantiomer is one of a pair of molecules that are non-superimposable mirror images of one another. The most popular way of visualising this is by taking a look at your hands: they are (more or less) mirror images of one another, but cannot be superimposed.
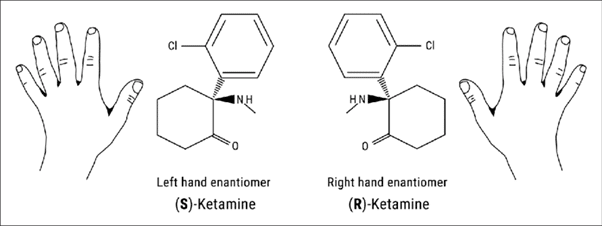
Janssen chose to develop esketamine, the left hand enantiomer in the figure above, for MDD, and were catalysed in this effort by a breakthrough therapy designation in the U.S., and fast-track status in Canada. Spravato was the subject of 29 clinical studies across 8 years prior to its approval.
Having conducted a great deal of clinical study at significant expense, and brought what is, prima facie, a novel therapeutic to market that purports to address an area of great unmet need, one might expect Janssen to benefit from data protection. This was the case in the U.S., at least, where Spravato was granted NCE status and thus awarded data exclusivity for 5 years (see the FDA’s Orange Book entry for U.S. patent and data exclusivity expiration dates).
Data protection generally affords a de facto monopoly over the marketing of a drug product to the first entity that commercialises a medicinal ingredient for any given indication. This prevents, for a given period of time, a generic version of the medicinal ingredient entering the marketplace. In Canada, this period of exclusivity is usually eight years.
The point of primary importance here is that only an innovative drug can benefit from data protection in Canada. According to the Food and Drug Regulations (FDR), an innovative drug is defined as:
“a drug that contains a medicinal ingredient not previously approved in a drug by the Minister and that is not a variation of a previously approved medicinal ingredient such as a salt, ester, enantiomer, solvate or polymorph“
Many of you will have spotted the issue for Spravato here: the definition of innovative drug, according to the FDR, explicitly excludes “variations of a previously approved medicinal ingredient,” which covers “salt, ester, enantiomer, solvate or polymorph” variants.
Given that ketamine hydrochloride was approved in Canada quite some time ago for use as an injectable anaesthetic, it was the Minister of Health’s opinion that esketamine hydrochloride—being an enantiomer of ketamine hydrochloride—was not eligible for data protection. In making this argument, the Minister cited Takeda, a case in which the drug DEXILANT (dexlansoprazole) was refused data protection due to it being an enantiomer of lansoprazole, a previously-approved medicinal ingredient.
Janssen sought a judicial review of the Minister’s decision with the Federal Court Trial Division, who upheld the Minister’s verdict noting that the “considerable effort” Janssen expended in its efforts to bring Spravato to approval and market were only a secondary consideration, with the stipulation of an “innovative drug” being a prerequisite to any such considerations of effort.
The precedent was of such relevance to Spravato’s case that Janssen sought to encourage the FCA to eschew its decision in Takeda, a departure the Court was unwilling to make due to the balast role of precedents in common law. Given that the Court did not view Takeda as “manifestly wrong,” the FCA upheld the Minister’s decision.
Now, the pharmaceutical juggernaut has again been denied by the courts; this time at the Federal court of Appeal (FCA). The appeal was dismissed unanimously, including by Justice Stratas who was in strong dissent at the Takeda case.
Janssen may attempt to appeal this decision at the highest level in Canada: the Supreme Court.They may also seek to have the case re-evaluated by the Minister of Health based on the fact that NAFTA, with which the prior regulations were compatible, has now been replaced by CUSMA.
Implications
It appears that, at least for now, it is not possible to obtain data protection in Canada for an enantiomer of a previously approved medicinal ingredient.
This is the case regardless of whether the generation of the underlying data entailed “considerable effort,” despite calls by some—including Justice Stratas in his dissent at Takeda—to take this into greater account where a drug contains an enantiomer of a medicinal ingredient that has previously received approval.
Now, many will naturally ask whether this is a green light for generic manufacturers to produce esketamine hydrochloride, which could potentially undercut Janssen’s pricing of its Spravato product.
If this were the case, this could have significant implications for the cost effectiveness of the therapy, which has been called into question by a number of groups.
Spravato’s Cost-Effectiveness
This isn’t the first time that Janssen’s Spravato product has butted heads with regulators, being denied after multiple rounds of discussion. The UK’s National Institute for Health and Care Excellence (NICE), which provides guidance to the country’s NHS with a particular focus on the cost-effectiveness of interventions and drugs, twice rejected to allocate NHS funds to Janssen’s esketamine nasal spray. Ultimately, NICE were not convinced by Janssen’s clinical data, and the product didn’t mesh with the Institute’s cost-benefit analyses.
In the US, meanwhile, a review by the Institute for Clinical and Economic Review (ICER) also took aim at the product’s cost basis, suggesting that it costs around $198,000 per quality-adjusted life year (QALY) gained. This sits well above ICER’s standard threshold of $150,000 per QALY. ICER also noted that its status as an enantiomer of low-cost ketamine “raises issues for all stakeholders about how to consider off-label prescription and coverage of a treatment that has not been as well studied but is being increasingly used for TRD.”
Hence, generic entry may make a nasally-delivered, fast-acting esketamine product more accessible to those who need it, at least for acute suicidal ideation or short-term treatment of depression.
It is important to note, however, that data protection is a different form of defence to patents, which are generally evaluated and defended separately. As such, any manufacturers seeking to market a generic version of Spravato would need to first evaluate Janssen’s patents on the matter.
Given that many psychedelics companies are looking to develop variations of known psychedelics, this news will likely raise questions among psychedelic drug developers and investors alike. Companies like Compass Pathways, for example, have secured patents on a number of variations of synthetic psilocybin, including polymorph and hydrate forms. Though, given that the medicinal ingredient in COMPASS’ lead candidate (COMP360), psilocybin/psilocin, has not previously been approved, the fact that the company holds patents over these synthetic forms, and the fact that the company appears the furthest ahead in their trials, may mean the company has less to worry about with this latest decision.
Other companies, meanwhile, may have more cause for concern: we will be watching this matter closely.
Australian University Strikes Deal Over Library of MDMA Analogues
Today, the University of Western Australia (UWA) announced that it has entered into an exclusive agreement with Emyria, a drug development and healthcare tech company, to explore the therapeutic potential of MDMA analogues.
Through the deal, Emyria gains rights over a library of over 100 MDMA analogues generated by Matt Piggott, an Associate Professor in medicinal chemistry at UWA. The announcement suggested an initial focus of the program will be on Parkinson’s disease, though details are sparse at present.
Thus far, Emyria’s drug development wing has focused primarily on cannabinoid-based medicines, targeting mental health and irritable bowel syndrome. The company appears to be engaged in preclinical work.
This announcement makes Emyria the latest in a growing crop of companies evaluating MDMA-like, or empathogenic/entactogenic, compounds. Matt Baggott’s Tactogen is also exploring this realm, as he explained in a recent interview with Erica Rex:
Among the characteristics he’s weeding out are the drug’s tendency to cause acute hypertension. MDMA increases heart rate. This is fine for a young healthy person, but less so for a person with a possible heart condition.
“Do we give Carvedilol against hypertension at the same time as we administer MDMA?” said Dr Baggott. Or is the solution to create a version of MDMA that does not affect heart rate and provides better safety for patients, even with less monitoring, a take-home preparation of MDMA. As it stands now, most of drug’s cardiotoxicity is dose dependent. The greater the dose, the more likely a person will suffer hypertension.
Speaking to Rex, Baggott also identified “loss of magic” as a limiting factor to one’s ability to gain benefit from MDMA, explaining that, “What we’d like to do is find a molecule that has the MDMA therapeutic effects [which don’t] decrease with repeated use.”
Atai Life Science’s EmpathBio, meanwhile, is developing EMP-01, an MDMA derivative for use in entactogen-assisted psychotherapy in conjunction with digital therapeutics to treat PTSD.
Cybin Begins Trading on NYSE
Yesterday, Cybin began trading on the NYSE American, under ticker symbol CYBN. It closed -13% on its opening day.
Other Headlines
- Awakn Life Sciences announces second UK location;
- Filament Health becomes first public company to be issued patent for extraction of natural psilocybin;
- Fireside Project releases app for psychedelic peer support;
- PsyBio adds NMDA receptor antagonists and associated analogues to IP portfolio;
- Psygen closes oversubscribed financing.
Weekend Reading
Fantastic Fungi Now Available on Netflix
Louis Schwartzberg’s documentary on mycelial connection, Fantastic Fungi, is now available on Netflix. The film, narrated by Brie Larson, has been described as such:
A descriptive time-lapse journey about the magical, mysterious and medicinal world of fungi and their power to heal, sustain and contribute to the regeneration of life on Earth that began 3.5 billion years ago.
Schwartzberg also announced the Fantastic Fungi Global Summit, a free virtual summit with conversations on ‘the power of fungi.’ The summit was also mentioned in Rolling Stone, who spoke to Deepak Chopra about the ‘virtues’ of psychedelics.
Weekly Bulletins
Join our newsletter to have our Weekly Bulletin delivered to your inbox every Friday evening. We summarise the week’s most important developments and share our Weekend Reading suggestions.
Live Updates
Join us on Twitter for the latest news and analysis.
Other Channels
You can also find us on LinkedIn, Instragram, and Facebook.


