2b, or not to be?
Preparing for COMPASS Pathways’ Data Readout
Heavily-anticipated results from COMPASS Pathways’ Phase 2b study of psilocybin-assisted therapy for treatment-resistant depression (TRD) are expected to be announced in the coming weeks.
In this special issue we explore the significance of these forthcoming results and aim to provide a primer on their interpretation.
A Psilocybin Alpha Special Issue
A Potential Sector-wide Catalyst
There’s a great deal of (nervous) anticipation around this study, not least due to it being the most advanced clinical trial assessing the safety and efficacy of psilocybin.
Company
Indication
Phase I
Phase II
Phase III
MAPS
MAPS is currently conducting Phase 3 trials investigating MDMA-assisted psychotherapy as a potential treatment of post-traumatic stress disorder after receiving Breakthrough Therapy Status from the FDA. More details about MAPS's MDMA product, including previous trial results, can be found in their published Investigator's Brochure.
The first Phase III study (MAPP1) was deemed successful and is published here. The clinical trial registration linked below is of a second Phase III study (MAPP2).
ClinicalTrials.gov Identifier: NCT04077437

Post-traumatic stress disorder (PTSD)
Compass Pathways
Compass Pathways' COMP360 is a psilocybin formulation currently being evaluated in a Phase II trial for potential use in treatment-resistant depression, after receiving Breakthrough Therapy Status from the FDA. The clinical trial registration linked below is of the ongoing Phase II study. COMP360 is also being evaluated in both preclinical and clinical (investigator-initiated) studies for numerous other indications.
ClinicalTrials.gov Identifier: NCT03775200

Treatment-resistant depression (TRD)
Usona Institute
The Usona Institute is currently evaluating psilocybin-assisted therapy in a Phase II trial as a potential treatment for major depressive disorder, after receiving Breakthrough Therapy Status from the FDA. More details about Usona's psilocybin product, including Phase I results, can be found in their published Investigator's Brochure. The clinical trial registration linked below is of an ongoing Phase II study.
ClinicalTrials.gov Identifier: NCT03866174

Major depressive disorder (MDD)
Above: Data from our Psychedelics Drug Development Tracker.
"A bellweather for a massive industry waiting in the wings"
This study is, for good reason, “a bellwether for a massive industry waiting in the wings,” according to Boris Heifets of Stanford University, who spoke to Psilocybin Alpha about the forthcoming results.
Heifets points out that if the study is a success, it will constitute the first positive published randomized placebo-controlled trial of psilocybin therapy for treatment-resistant depression.

Expert Spotlight: Boris Heifets
Trial Design & Context
This isn’t the first study of psilocybin-assisted therapy, but it does differ in important ways to earlier work. Notably, it adheres to the rigorous standards required by the U.S. FDA to proceed through the clinical trial, and ultimately approval, process.
The trial compares three doses of COMP360, the company’s proprietary psilocybin formulation of synthetic psilocybin, in over 200 patients across the world. Participants in the trial are suffering from treatment-resistant depression, meaning they have failed to respond to conventional therapies. This is thought to represent around a third of patients with major depressive disorder (MDD).
Thus far, studies of psilocybin-assisted therapy have evaluated broader indications such as depression and anxiety in the context of a cancer diagnosis (see Grob et al., 2011, Griffiths et al., 2016, and Ross et al., 2016) or lacked adequate placebo control groups (see Carhart Harris et al., 2016 and Davis et al., 2020). Despite the inherent difficulties in employing a placebo control in trials of psychedelics, they are necessary to progress through the clinical trial gauntlet.
Carhart-Harris et al.’s highly-anticipated 2021 study published in the New England Journal of Medicine, which pitted psilocybin against escitalopram, represents the most rigorous trial of psilocybin therapy’s antidepressant effect to date. While encouraging results can be found in the supplementary materials, the study failed to show a significant benefit of psilocybin over escitalopram on its primary measure.
While Heifets, perhaps in an instance of self-deprecation, admits that these differences may be viewed as “pedantic hair splitting” (a view this author certainly doesn’t hold), he told Psilocybin Alpha:
“Even if Rolling Stone, Newsweek and 90% of Twitter proclaim the transformative power of mushrooms for mental health, the FDA will have to evaluate the evidence for whether psilocybin therapy has a benefit compared to placebo (or other treatment) for a specific diagnostic indication [such as TRD]”
Such is the task ahead: convincing the FDA, and in turn other national drug regulators, that psychedelic-assisted therapies (and psychedelics themselves, as in early-stage drug development initiatives that don’t see the only role of psychedelics as a catalyst to psychotherapy, or target non-psychiatric indications altogether) are safe and effective. This must be achieved through accepted, rigorous clinical trial pathways, which were – to some extent – the cause for the original winding-down of psychedelic research a half-century ago.
Risk-of-bias Asssessment
Psilocybin Alpha Pharmaceutical Advisor Michael Haichin, PharmD, BCMAS, further elucidated the differences between the study under discussion and earlier studies of psilocybin-assisted therapy.
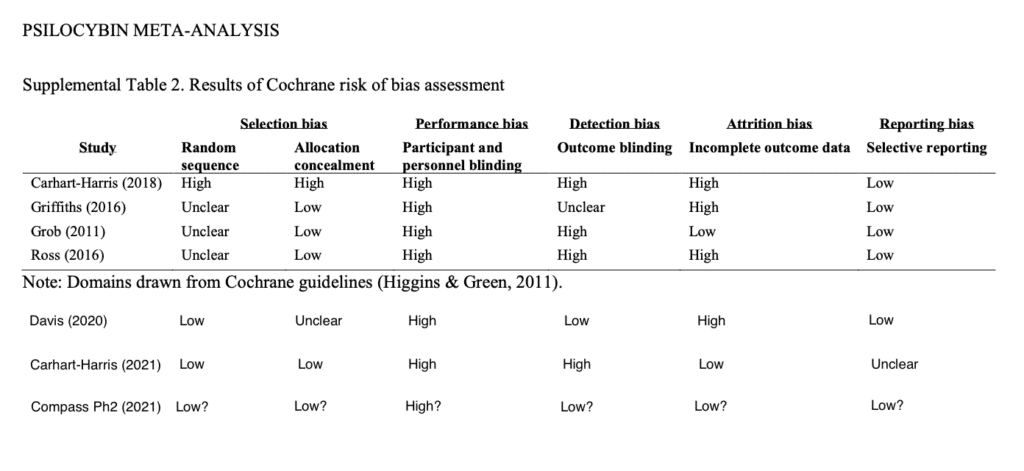
Haichin sought to bring the work of Goldberg et al. 2020 up-to-date by adding three recent studies, and conducting Cochrane risk-of-bias assessments for each. As you can see, if COMPASS does things correctly as anticipated, they will have reduced the risk of bias in some areas such as in outcome blinding which is achieved by using independent, blinded outcome assessors (further note: Eduardo Schenberg made an important comment on this via the Twitter thread: “Triple blinding is very helpful. But it also must be measured, to know if blinding worked or not, as assessors may unblind while interacting with patients.”). However, as with most psychedelics studies some areas of blinding are perhaps impossible, as represented in the participant and personnel blinding column.
Setting the Goalposts: What Defines ‘Success’?
As we await the publication of these results, what should we be looking out for? What constitutes a ‘success’?
According to the study’s ClinicalTrials.gov entry (catalogued in our Psychedelics Drug Development Tracker), the primary outcome measure for this trial is the Montgomery-Asberg Depression Rating Scale (MADRS): a commonly-used clinician-rated scale used to measure the severity of a participant’s depression.
So, when the Phase 2b data is provided we will be looking to see a clinically and statistically significant change in depression – as represented in MADRS ratings – between at least two of the three psilocybin dosage groups (low, medium, high).
Heifets and others believe that it is likely that COMPASS will compare the (presumably) sub-therapeutic ‘low’ dose (1 mg) with the high dose (25mg; see Garcia-Romeu et al., 2021 for a discussion of weight-adjusted versus fixed dosing approaches) when attempting to demonstrate statistical significance in the reduction of depression severity. Statistical significance in this case might suggest the benefits of psilocybin-assisted therapy for TRD.
But, Heifets reminds us not to discount the medium dose entirely. Having dug through the supplemental data in Carhart Harris et al. (2016) – an open-label trial of psilocybin for MDD – the Stanford professor explains that “most of the therapeutic benefit” observed in the trial “happened after the medium (10mg) dose;” a dose also apparently effective for migraine headaches Schindler et al., (2021), he notes.
HAM-D data for weeks following each dose
Baseline = 21.4 ± 4.5
One week post low dose = 10.7 ± 7.8
One week post high dose = 7.4 ± 6.9
Supplementary data from Carhart-Harris et al. (2016).
This leads Heifets to question: “What if the medium dose and high dose are both superior to the low dose, but indistinguishable from each other?”
This would certainly be an interesting result. If psilocybin is a “placebo enhancer,” for example, Heifets suggests that a medium dose may be sufficient to trigger that effect. He goes on to explain that an appropriate statistical analysis plan should account for this possibility.
If the medium dose does appear to be as effective – or at least similarly effective – to the high dose, that would be a significant finding that may have implications for the approval and roll-out of the therapy. Heifets indulged briefly in such speculation, before noting that other researchers claim to have correlated the intensity of the subjective experience with the transformative or therapeutic benefit (see Yaden and Griffiths, 2021).
There’s no need to get too bogged-down in these complexities, however. COMPASS Pathways will publish the topline results in a press release, and we will parse out the key takeaways here on Psilocybin Alpha.
A Rising Tide, or Another Damp Squib?
As investors, analysts and advocates across the board appear to be expecting positive results in the coming weeks, should we understand this as a potential inflection point for the entire space?
Analysts are expecting positive results to cause a sector-wide rally, while negative or weak results could fail to stem the slow decline in stock prices many companies in the space have seen this year: COMPASS Pathways, for example, was down 27% from its December ’20 high at the end of October. Atai Life Sciences, which owns approximately a fifth of COMPASS, was also down 22% from the price it scored shortly after its June ’21 IPO.
The sector more broadly has taken a haircut for most of this year, with notable ETFs tracking the top public companies in the space dropping by up to a third since Spring. Hence, many are hoping this event will raise the basket of stocks.
As a case-in-point of the sector’s excitement around this data readout, MindMed’s CEO Robert Barrow told Psilocybin Alpha:
We are extremely excited about the readout from this study and believe that positive results of any magnitude and of any of the tested doses will be a boost for the entire field. Psilocybin and related drugs offer an important first assessment of the clinical activity of serotonergic psychedelics in sponsored clinical trials, setting the stage for more psychedelic therapies to progress through clinical development. Any results that are both clinically and statistically significant will draw attention to the promising research being done in this space.
Robert Barrow, MindMed CEO
However, atai Life Sciences’ IPO in June of this year was also heavily anticipated, with most commentators expecting the event to have a positive effect on stock prices across the sector. However, these expectations were largely unmet, with most companies trading flat or lower at market close the day of atai’s listing, and since.
But, one might argue that the forthcoming results from COMPASS’ Phase 2b study are fundamentally more significant for the future of this nascent space, in contrast to a financial event like atai’s IPO. If successful, COMPASS’ Phase 2b study would serve to validate a whole host of earlier-stage clinical trials of serotonergic psychedelics. Remember: the only Phase 3 trials of ‘psychedelics’ at present relate to ketamine and MDMA, both of which evade the true definition of the class of drugs.
Whatever happens, it’s likely to be volatile. Bloomberg’s analysis of CMPS options suggested that the stock could move 57% in either direction by mid-December, with ATAI potentially moving 29% either way.
The old adage of buy the rumour, sell the news, may also ring true. With nearly a dozen Wall Street analysts handing a buy rating to the stock already, it’s not the case that COMPASS is off the radar. At the time of writing (4th November, 2021), the stock is up 53% in the past month, with many other psychedelics stocks like MindMed (MNMD) also enjoying a raise in the past couple of weeks.
Double-down or Pivot Away?
It’s most likely futile to speculate on what COMPASS Pathways might choose to do should the readout show either positive or negative results, but this author can’t help himself.
If the readout shows clinically and statistically significant results for the company’s COMP360 protocol, we should expect COMPASS to continue pushing this drug development program through to Phase 3, with an eye on being the first company to secure FDA approval for psilocybin-assisted therapy.
It’s likely that the British company would also use such a positive announcement to raise further funds, with a shelf prospectus for a raise of up to $150m already on the books with the SEC. Additional funds, if they don’t come from a significant bump in the stock price, might also be directed to targeting other indications such as PTSD, which we briefly discuss below.
Should the results fail to show a clinically and statistically significant benefit of COMP360 in the treatment of TRD, it’s difficult to imagine how the company may respond.
Perhaps the company will pivot further toward the development of ‘psychedelic 2.0’ drug candidates, like the work being conducted by Jason Wallach and Hamilton Morris at USciences (note: Hamilton Morris recently appeared on Psychedelics Today’s podcast, during which he discussed this work).
The Bottom Line: 2b, or not to be?
While acknowledging that the success rate of drugs in Phase 2 clinical trials is dicey (approximately half of CNS drugs that make it to Phase 2 graduate to Phase 3), we don’t see significant reasons to expect especially poor results in this study.
Some investors were spooked by the filings of Form 144s by COMPASS founders George Goldsmith and Ekaterina Malievskaia in August. According to the SEC, a person filing a Form 144 must have a bona fide intention to sell the shares referred to in the Form within a reasonable time after filing it. This is understood to be a three-month period, which would allow the founders the opportunity to sell a portion of their shares in the company up until mid-November, given the filing was made in mid-August. However, it must be noted that these proposed share sales represent a relatively small portion of the founders’ shares.
On the contrary, some have pointed to the company’s recent announcement of a psilocybin-assisted therapy for PTSD program as a more bullish signal. The planned Phase 2 trial will employ the COMP360 protocol used in the TRD study, perhaps implying the company’s confidence in the paradigm.
Either way, the results of this study are an important moment – and potentially an inflection point – for this nascent industry.
You can be sure that we will be among the first to cover the data readout, whenever it may appear.

