A Dose of Realism?
I have had the pleasure of attending many psychedelics conferences (and psychedelic ‘tracks’ of larger conferences) over the past few years. They’re usually a little different to your standard conference; a little weird.
Hot off the heels of SXSW in Texas, I headed to the European College of Neuropsychopharmacology’s (ECNP) New Frontiers meeting in Nice, France. This year’s two-day meeting was dedicated entirely to psychedelics.
The mood couldn’t have been more different to that seen at SXSW, which—in spite of the SVB collapse—had a very optimistic tenor, with techno-futurist or even transhumanist undercurrents palpable at many of the talks. This extended to the psychedelics talks and panels which, despite purportedly ‘going beyond the hype’, were still largely positive and optimistic. Everything’s bigger in Texas, they say.
In this context, ECNP was a dose of European realism. From the anticipated effect sizes of psychedelic trials through to the cut and tailoring of suits and outfits—everything felt a little more measured, here.
It was a decidedly academic crowd, with the vast majority of attendees hailing from the world of research. As I milled around the hallway of Le Méridien hotel, where a temperamental coffee machine provided fodder for the always-slightly-awkward cold introductions, I couldn’t help but feel like there was something different about these researchers than their North American counterparts.
I think it’s something like this: the academics I met at ECNP didn’t strike me as typecast ‘psychedelic researchers’, but rather ‘researchers that do some work on psychedelics’1. Perhaps it’s due to the fact that there are fewer psychedelic research centres in Europe, meaning researchers are primarily embedded in—and answerable to—their respective fields and departments?
This hypothesis is nested within larger differences in the funding streams most readily available to researchers interested in psychedelics in Europe. Aside from company sponsored studies, psychedelic research in the U.S. is often funded by psychedelic-aligned philanthropists2.
Others I spoke to suggested that this difference in funding might also influence the type of research that we see. Might this explain the fact that there appears to be more3 research on adverse events4 (including challenging or difficult experiences) in Europe?
Maybe it’s also to do with how clinical psychedelic research is simply further along in North America. Both MDMA and psilocybin received Breakthrough Therapy Designations from the FDA years ago, while European regulators like those in the UK have only recently issued similar designations.

“We don’t know.”
The meeting’s aim (and, indeed, that of ECNP more broadly) was to bring together academia, regulators and industry to discuss the future of psychedelics. ECNP’s dedicated Psychedelic Research Thematic Working Group, meanwhile, has a number of specific goals that range from theoretical to concrete: from supporting policymaking and examining mechanisms and pathways of psychedelic action through to investing in shared stocks of GMP psychedelics and sharing data.
The meeting’s agenda was split into a number of keynotes and sessions including preclinical pharmacology, clinical aspects of psychedelic research, and regulatory considerations. While there was a small amount of unpublished data shared5 (including Timmermann et al.’s DMT brain imaging study, which was released the next day), a significant portion of the discussion revolved around what we don’t know.
The most common phrase I heard uttered by speakers and panellists across these sessions was, “we don’t know”; usually followed by some explanation of the type of research that might bridge the knowledge gap they described.
These gaps and questions include (but are certainly not limited to):
- A more thorough understanding of the interactions between antidepressants like SSRIs and psychedelics.
- A reliable separation of the therapeutic effect of the psychotherapy versus the psychedelic.
- How to ensure and maintain blind; or, how to measure the blind and adjust data accordingly.
- The optimal duration of a trip.
- Will non-hallucinogenic psychedelics (psychoplastogens) be effective?
- Why do some patients experience enduring benefits, while for others beneficial effects appear to wear off quickly?
- What is the optimal number of dosing sessions, and timing?
- The search for preclinical assays with reliable translatability to humans.
On the topic of these gaps, David Nutt suggested that more preclinical research is needed. The preclinical side of the field needs to “catch up” to the clinical, he said.
Challenges for Psychedelic Drug Development
The varied presentations and lengthy Q&A sessions provided ample time to explore various challenges that psychedelic researchers and trial sponsors may face, as well as discussing potential solutions.
Learning from Spravato
Steffen Thirstrup, CMO of the European Medicines Agency (EMA), told the audience that Spravato’s drug development and approval process might provide some interesting lessons learned for psychedelic drug developers6.
Thirstrup introduced Jaskaran B. Singh, a former Senior Director, Neuroscience at Janssen, to share some thoughts on the esketamine clinical development program that he was part of. Altogether, Janssen’s esketamine product went through nineteen Phase 1, four Phase 2, and six Phase 3 studies; meaning the safety of esketamine was evaluated in over 1,700 patients.
Two of the company’s Phase 3 studies—TRANSFORM-1 and TRANSFORM-3—were “negative”7, Singh explained. The earliest studies of the drug had the largest effect sizes, then it “sort of went downhill from there on”, he said, explaining that the fact that effect sizes tend to reduce over time is “largely because of variability in your control arm.”
Singh went on to share his advice for psychedelic drug developers and clinical trial sponsors. His top three lessons learned from esketamine’s Phase 3 program were: “site selection, site selection, site selection.” He added: “How do you select sites? The shortest answer is, get hold of every resource possible because you can’t learn enough.”
Increasing recruitment is a significant challenge for such trials, Singh explained, despite the fact that almost all stakeholders involved in a trial will be pushing for it. This can be achieved via a four pillar strategy which includes a central ad campaign, local recruitment, engagement with consortiums and via the enhancing of site relations. Singh suggested the latter—enhancing site relations—is especially important for products like Janssen’s esketamine nasal spray and, in turn, psychedelic therapies, with sites needing a lot of support in these cases.
Singh also suggested that underperforming sites should be replaced if necessary, pointing to the example of Relmada Therapeutics where a handful of sites appear to have generated enormous issues for the company in its Phase 3 trials of NMDA receptor channel blocker depression drug REL-10178.
Just yesterday, in fact, Relmada shared via its Q4 2022 update that it had learned from these failures, and resolved that “[f]rom the extensive analyses of Study 301, we now know how to identify the most reliable sites, the most suitable patients, and greatly improve our study protocols.”
The impact of assessments and “getting the right patients”, Singh noted, is critical. As Janssen implemented closer quality monitoring of patient interviews and assessments9, the effect of esketamine became a lot clearer, separating from placebo more convincingly.
Singh also had other suggestions, such as attempting to decouple study participation from ‘outcome’ by making the investigational drug available to all patients via an extension arm; otherwise, patients may drop-out of the study if they guess that they are in the placebo arm, or bend any criteria that might gate entry to an extension arm. This design is already used by some psychedelic drug developers, including in COMPASS Pathways’ Phase 3 program.
Some of the challenges faced in the development of esketamine will only be more pronounced in psychedelic drug development. Take, for example, the issue of expectancy. “Publicity is great for raising funding”, Singh said, “but it really impacts patient expectations”. Given that psychedelics are receiving even more publicity than Janssen’s esketamine was during its trials, we should expect to continue seeing significant expectancy which might impact the placebo and nocebo response.
EMA’s Marion Haberkamp, a Member of the Central Nervous System Working Party (CNSWP) also leveraged Spravato as a case study to provide a European regulatory view on psychedelic drug development.
Haberkamp explained the elements of the esketamine study designs that allowed EMA to consider Janssen’s efforts to minimise the risk of a potential effect on blinding to be considered sufficient. These include independent, blinded, remote telephone MADRS assessment, different raters performing efficacy and safety assessments in the Phase 3 studies, and the addition of a bittering agent to the placebo solution to simulate the taste of the esketamine solution in the nasal spray device.
In terms of lessons learned from the Spravato case study, Haberkamp said that the timing of the primary endpoint is important: EMA wants to see maintenance of effect, not just short-term efficacy. She explained that regulators will be very interested in how long the effect will last, and when/whether conventional antidepressants can be reintroduced. The need for retreatment should also be investigated, she added.
“These compounds pose new challenges for regulators”
On the topic of psychedelics, Haberkamp explained that this class of drugs “pose new challenges for regulators”.
The challenges that psychedelic drug developers face, Haberkamp explained, can broadly be separated into scientific, legal and implementation-related. Expectancy bias—both positive and negative—is among the main challenges, according to Haberkamp, as mentioned by other presenters and panellists throughout the meeting.
Second up is the “placebo issue” and maintaining the double blind. Elsewhere in the meeting, researcher Gerhard Gründer said that “it’s not really possible to blind”, and FDA’s Tiffany Farchione told participants that “there needs to be some effort made to reduce those biases and to provide a valid comparison to something… if you can’t eliminate the bias you at least need to account for it somehow.”
Other challenges identified by Haberkamp include dose finding; demonstrating maintenance of effect; teasing out psychotherapy’s or psychological support’s role; the standardisation of setting and training; safety; and, HTA and post-approval considerations.
But, drug developers need not navigate these challenges alone, at least with regards to the EMA. Haberkamp was keen to point out statistics on uptake of the EMA’s scientific advice facility, which show a very low level of interaction between EMA and drug developers in the psychiatric diseases category versus, say, infectious diseases.
“Scientific Advice and early dialogue is recommended and is probably helpful”, her slides read. More psychedelic drug developers might be wise to take up the EMA on its offer of advice!
Haberkamp ended by mentioning the forthcoming revised EMA Depression Guideline, for which she is the lead drafting editor. It will include a chapter on psychedelics.
Tiffany Farchione followed Haberkamp’s talk by providing the FDA perspective. Farchione also identified placebo control as a challenge for psychedelic trials, and noted that a variety of factors complicate the assessment of efficacy in such trials including: an engaged practitioner; patient expectations (on this point, Farchione’s slides showed a picture of the cover of Michael Pollan’s best-selling book, How to Change Your Mind); the elaborate nature of the intervention; ‘dramatic’ functional unblinding; and, hypersuggestibility.

Farchione explained that, in order to meet the valid comparison requirements, sponsors might explore alternatives to inert placebos (which are not only ineffective due to functional unblinding, but may precipitate a nocebo effect). This could include using active placebos, dose-response trials, or factorial designs, which could be very beneficial in understanding the contributions of drug and non-drug effects. ‘No one is really doing this’, she said on the topic of factorial study designs.
FDA also expects sponsors to implement measures to minimise bias, and to assess the impact of potential biases. These could include blinding questionnaires or videos of raters to assess inter-rater reliability, for example. Another suggestion from the FDA for how to reduce potential biases is to have the post-treatment therapist be different from the in-session monitor. More on that below.
Elsewhere, Farchione reminded the audience that FDA’s Risk Evaluation and Mitigation Strategies (REMS) are drug safety programs: they are designed to prevent, monitor and manage serious risks. She was keen to point out that they are not intended to assure effectiveness: “they are strictly a safety program”.
As mentioned by other speakers, including EMA’s Haberkamp, FDA would like to understand the durability of response to inform timeframes for repeat dosing, if any, and hope to see sponsors following (in an open label extension period) subjects for six months to a year.
Where Regulators and Researchers Disagree
One of the points raised by FDA’s Farchione got me thinking. As aforementioned, Farchione suggested that sponsors might reduce potential biases by having post-treatment therapy conducted by a different person than the in-session monitor.
This approach struck me as out of kilter with what a number of researchers had mentioned throughout the meeting, prior to Farchione’s presentation.
Gerhard Gründer, who leads a psilocybin for depression study funded by the German government (see our November 2020 coverage), believes that “if you administer a psychedelic there’s a special relationship between the therapist and the patient”. Gründer shared emails10 from two patients in his trial to illustrate his point, with one patient writing: “I am infinitely grateful to you, […] I cherish you in deep friendship, not as therapists but as people.”
The most important factor in the efficacy of psychotherapy is the alliance between patient and therapist(s), he said, adding that “we may lose efficacy” if we separate the therapists who psychologically support the administration of psychedelics from “true psychotherapists.” Gründer was emphatic: this alliance “cannot be delegated for a couple of days to someone to administer the drug”.
Elsewhere, during a question and answer period, David Erritzoe discussed how the responsibility of the therapist in managing the very difficult (and sometimes possibly false, dream-like) material that may emerge during a psychedelic session is “immense”, “massive”.
Upon hearing the FDA’s proposal of having a different person conduct the post-drug integration sessions, I wondered how this might work given Erritzoe’s experiences. How might a therapist who did not witness the psychedelic session take on this “immense” task?
Perhaps this is why psychedelic drug developers and researchers alike have not adopted this element in their study designs.
This mismatch between FDA advice and our current (presumed) ‘best practice’ of psychedelic therapy perhaps points to a larger piece of context that is always worth remembering: the FDA does not regulate psychotherapy. Given this fact, it’s not surprising (or sinister) that the agency is looking to disaggregate the drug effect from the psychotherapy, or psychological support.
It does continue to generate friction and challenges, though, for psychedelic drug developers who walk a fine line between satisfying the FDA that they have taken adequate steps to reduce bias and provide a valid comparison to something, and maintaining key elements of psychedelic-assisted therapy that are presumed to drive its safety and efficacy.
Gründer’s advice (or, plea) to regulators: “Don’t just look at curves. Listen to the patients and to the therapists!”
Miscellaneous Notes on Other Topics
Matthias Liechti made an interesting comment on his lab’s candyflipping study, which sees the co-administration of MDMA and LSD. When it announced the commencement of the study, the trial’s sponsor, MindMed, hoped that the MDMA might increase ‘positive’ subjective effects and reduce ‘negative’ subjective effects that may be associated with LSD.
At the ECNP meeting, Liechti shared that he doesn’t believe the coadministration of these two drugs necessarily has a synergistic effect. Rather, he proposed that the fact that MDMA is a CYP2D6 inhibitor simply extends the subjective effects slightly.
On the topic of the interaction between SSRIs and psychedelics, Liechti explained that a forthcoming study will see patients pre-treated with SSRIs for six weeks (as opposed to the two week pre-treatment period in their prior study), and David Erritzoe mentioned that MAPS is investigating whether it could allow patients to continue taking SSRIs but administer a higher MDMA dose to account for any blunting of the psychedelic effect.
Erritzoe also mentioned that there are some interesting trials publishing soon on the topic of microdosing. Current data is at the low end of the hierarchy of evidence, he said, but added that microdosing gives us the opportunity to properly blind participants. Also on the topic of microdosing, Nutt said, “it’s probably safe”. Presumably he is not concerned about the potential risks related to chronic 5-HT2B agonism, which have been discussed elsewhere by a number of researchers.
On the topic of managing patient expectations, Katrin Preller urged colleagues to be very honest with trial participants. “Managing expectations and being very transparent is important”, she said, explaining that patients should be told that psychedelics won’t provide “magic relief” from depression or alcohol use disorders, for example. Rather, they should be reminded that it’s a process: “it requires work, it requires a patient therapist relationship.” Researchers might also tell patients, “don’t be disappointed if it’s not a mystical or magical experience.”
Gerhard Gründer made a number of interesting comments on effect sizes. He said that effect sizes are likely to be much larger where primary endpoints are shorter, using the example of a recent psilocybin for major depressive disorder (MDD) study (von Rotz et al., 2023) which had a primary endpoint at 14 days after the psilocybin session. Gründer’s group chose to measure the primary endpoint for its psilocybin for depression study at six weeks.

Gründer said that COMPASS Pathways’ Phase 2b results were, “much more realistic than what the smaller studies show”, with response levelling out and only “around 30%” seeing remission11. This is “more realistic and what we see in our large trial”, he explained.
Taken together with Singh’s comments, we might expect effect sizes to diminish significantly as we evaluate psychedelics in later stage trials and across longer endpoint timelines.
Tomáš Páleníček discussed ayahuasca ceremonies, shared a comparison between EEG and fMRI data looking at acute and persisting effects (forthcoming), and hypothesised that, aside from the peak experience during a psychedelic administration session, it might be important to look at where the participant finds themself at the end of the session. He also showed some data on music perception during a psychedelic session, with American industrial metal band Ministry performing poorly!
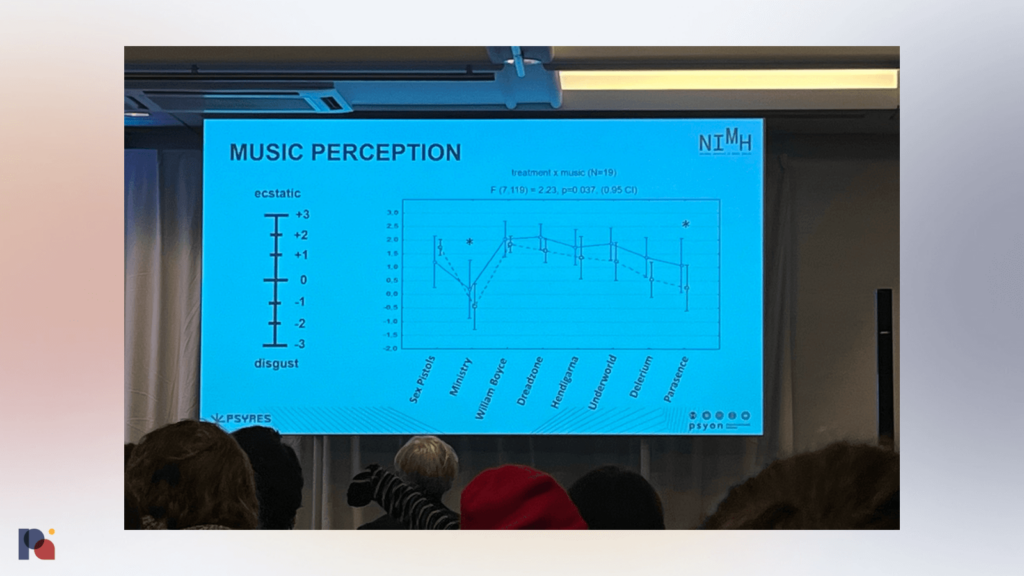
Páleníček ended his talk by playing footage from an ayahuasca ceremony, to which EMA CMO, Steffen Thirstrup, light-heartedly and rhetorically asked how we might get some of those elements into a product label in Europe.
Drummond E-Wen McCulloch, who is also the Scientific Secretary of ECNP’s psychedelic research working group, shared some natural language processing (NLP) analysis of qualitative reports. According to the analysis, words that most commonly characterised the mystical experience included “universe”, “Dad”, and “beautiful”. Interestingly, among the top words was “magnetic resonance”.
Patients were undergoing an MRI scan during their psychedelic experience, which to many might sound like a miserable setting for a trip. But, one participant explained: “I felt a sense of no longer being connected to my own body. The MRI scanner and I stepped into a different reality together. With colours and shapes and figures.”
McCulloch also shared a trial design that will manipulate the setting of the psychedelic experience, with two different preparation sessions and two different acute settings during the administration session (music vs. no music). He hopes that this will help us get at the question of whether the acute and persisting effects of psychedelics are causally related.
The meeting closed with a talk on the patient perspective led by Tadeusz Hawrot, who runs the Psychedelic Access and Research European Alliance (PAREA). After a short overview of his organisation, and the importance of hearing the patient perspective, Hawrot led by example as he screened a short patient testimonial video from an MDMA-assisted therapy for alcohol use disorder participant, Dave Pounds.
Jules Evans of the Challenging Psychedelic Experiences Project then addressed the audience via Zoom, during which he shared his own challenging psychedelic experiences and explained that he hoped to see more into the potential risks and negative side effects of psychedelics.
There was much more discussed that isn’t captured in this short (and largely unorganised) dispatch, including discussion on non-hallucinogenic psychedelics (which featured some back-and-forth between David Nutt and David Olson, who appeared via Zoom); a presentation by Emma Robinson that presented her work on how affective bias might be used as a cognitive biomarker; plenty of neuroimaging presentation and discussion; and much more.
Fortunately, ECNP is expected to publish one or two pieces which will more comprehensively capture the presentations and discussions that took place at the two-day meeting. Stay tuned.
Weekly Bulletins
Join our newsletter to have our Weekly Bulletin delivered to your inbox every Friday evening. We summarise the week’s most important developments and share our Weekend Reading suggestions.
- You really should read Matt Zorn’s thoughts on how and why he does not identify as a psychedelic lawyer for an analogous distinction—it represents the prior art for my thinking here.
- A pair of European researchers told me that their colleagues have looked to the foundations of car manufacturers like BMW and Volkswagen for funding, to little avail.
- See, for example, ICEERS’ Support Center, Jules Evans et al.’s Challenging Psychedelic Experiences Project, and publications from the likes of Breeksema et al. (2022) and Simonsson et al. (2023).
- At the meeting, Gerhard Gründer made the point that we need consensus on how to define these events: i.e., whether they should only include instances where medical attention or intervention was required.
- Which, in the spirit of collegiality, is not reproduced herein.
- I have previously drawn attention the lessons that psychedelic drug developers might learn from health technology assessments of Spravato.
- Singh’s slides preferred the terminology, “Not Statistically Positive”, though he opted for “negative” when speaking.
- Note that Relmada marked up the failure of its first two Phase 3 trials as being due to an ‘implausible placebo response’. atai’s PCN-101 candidate, also an NMDA receptor antagonist in development for depression, has struggled in trials, with some pointing to a large placebo response. Large placebo responses are an issue for psychedelic drug development more broadly.
- Singh said that the implementation of audio recording to allow for independent MADRS assessments significantly improved rating quality.
- The full text of the emails showed that the patients are remarkably eloquent and proficient in expressing their feelings. This makes me wonder about selection bias; though another interpretation might be that psilocybin-assisted therapy is an antidote for alexithymia!
- Gründer also asked whether “remitted” patients can be considered “healthy”, given the fact that health-related quality of life (HRQOL) among remitted patients is often lower than that of the general population. He prescribed long-term studies of psychedelic-assisted therapy outcomes, and comparisons of psychedelics to standard of care (not just placebo). This is what health technology assessment agencies and payors will likely require, afterall.


