Below, we discuss CB Therapeutics’ patent application that covers methods of biosynthetically producing psilocybin. We also touch on mescaline, which MindMed is set to investigate in a Phase 1 trial.
In the Weekend Reading section, we share fMRI data from two psilocybin trials and highlight a recent BBC documentary titled The Psychedelic Drug Trial.
Psychedelic Sector News
CB Therapeutics’ Patent Application Met with Questions
Two days ago, a new patent application by CB Therapeutics was published, as first noted by our editor-at-large Graham Pechenik on Twitter. It claims priority to a provisional filed on November 15, 2019, and was announced in a press release the following month.
The patent application, broadly, covers genetically engineered host cells that produce psilocybin. The first claim in the application reads as follows (emphasis our own):
Claim 1: A recombinant host organism comprising: a plurality of cells transfected by a set of genes for synthesizing psilocybin in the recombinant host organism via at least a first pathway and a second pathway; wherein the recombinant host organism is a fungal species comprising: Schizosaccharomyces cerevisiae, Schizosaccharomyces japonicus, Schizosaccharomyces pombe, Schizosaccharomyces cryophilus, Saccharomyces cerevisiae, Kluyveromyces lactis, Kluyveromyces dobzhanskii, and Yarrowia lipolytica; wherein the set of genes comprises any combination of a gene selected from a group consisting of PsiD, PsiH, PsiK, and PsiM.
Just over two years prior, Fricke et al. published Enzymatic Synthesis of Psilocybin in the journal Angewandte Chemie (Applied Chemistry). In this paper, Janis Fricke, together with Felix Blei and Dirk Hoffmeister, characterized the enzymatic basis for the biosynthesis of psilocybin—as a reaction involving the four enzymes PsiD, PsiH, PsiK, and PsiM.
The article concluded:
“Our findings set the stage for heterologous production of [psilocybin] in a controlled place for pharmaceutical purposes, using engineered microbial hosts, should the re-discovered pharmaceutical value lead to increased demands.”
Just last week CB Therapeutics announced the closing of an oversubscribed Series A filled entirely by re.Mind Capital, a fund that sits within Christian Angermayer’s family office. As we commented in last week’s bulletin, Atai Life Sciences’ CSO joined the board of directors, as did a founding partner of re.Mind. Angermayer, described as ‘the psychedelics kingmaker’ in the press release, joined as an advisor.
Some have expressed concern at this latest development. James Keim, CEO of Mimosa Therapeutics, wrote on Linkedin:
The original paper from which CB seems to have borrowed without attribution is by Dr. Janis Fricke, Dr. Felix Blei (currently VP for Analytics and co-founder of Mimosa Therapeutics and founder of MIRACULIX), and Dr. Dirk Hoffmeister. Fricke and his colleagues had decided to publish rather than patent this technology in his best effort to make sure that this gift would not become a restrictive patent.
In fact, this is not the first time that a patent application has been filed on the biosynthetic production of psilocybin using these enzymes. A team at Teknologian Tutkimuskeskus Vtt Oy (The VTT Technical Research Centre of Finland) has also filed a patent application, entitled “Heterologous production of psilocybin.” This application, currently pending in the U.S., Canada, and Europe, claims priority to March 19, 2018, and was published as a PCT application on September 26, 2019 (see WO 2019/180309A1).
The first claim in the published PCT application reads (emphasis added):
Claim 1. A recombinant host cell comprising: heterologous polynucleotides encoding PsiD, PsiH, PsiK, and PsiM; wherein the heterologous polynucleotides are operably linked to at least one promoter which is capable of directing expression of said heterologous polynucleotides in the host cell; and wherein the recombinant host cell is capable of producing psilocybin.
(In the background, the application notes that these “[g]enes responsible for biosynthesis of psilocybin in basidiomycete mushrooms [were] identified and disclosed in a paper by Fricke et al. (2017).”)
CB Therapeutics’ new patent application raises a number of questions, including:
- Is simply putting work into the public domain sufficient to prevent it from later being patented?
- Is there something novel and non-obvious that distinguishes CB Therapeutics’ patent application from the earlier published work of Fricke et al. and VTT?
- Will we see objections to this patent application?
- Of course, will the patent be granted?
- And if granted, what might it mean for others also working to produce biosynthetic psilocybin in yeast, including companies such as Octarine Bio (see our November interview with the co-founder and CSO here)?
We will be following the course of this patent application closely, and you can track it and all other psilocybin-related applications in our Psilocybin Patent Tracker.
MindMed to Explore Mescaline in Phase 1 Trial
This week, MindMed announced ethics committee approval for a Phase 1 clinical trial evaluating the acute effects of mescaline across 6 different dosing conditions.
While it is already known that the threshold dose for mescaline is far higher than that of psilocybin and LSD, MindMed is seeking to understand dosing effects in the context of a clinical trial. MindMed President Dr. Miri Halperin Wernli said:
With our rigorous clinical trial, we aim to characterize the subjective effects of different doses of mescaline and provide a description of the acute mescaline effects to help clarify the involvement of the 5-HT2A receptor in mescaline-induced altered states of consciousness in healthy people.
Like other classical psychedelics, we know that mescaline binds to the 5-HT2A receptor with a high affinity. MindMed will attempt to better understand the involvement of the 5-HT2A receptor by administering ketanserin, a 5-HT2A antagonist, prior to mescaline.
The company has previously described ketanserin as an LSD neutralizer technology, but—despite filing a patent application on the topic—is yet to demonstrate that administering the drug during LSD therapy is successful in stopping a trip (more here). The use of ketanserin in this mescaline study will not attempt to demonstrate its efficacy in stopping a mescaline trip, either.
The trip occasioned by mescaline is very lengthy, averaging around 10 hours. Meanwhile, many companies pursuing psychedelic drug development are seeking to identify shorter-acting psychedelics. In this sense, MindMed’s exploration of mescaline runs counter to this industry trend.
MindMed hopes to begin the trial later this month.
Further Reading on Mescaline
Mescaline is a phenethylamine found in a number of cactus species, including peyote (Lophophora williamsii) and San Pedro. Aside from being popularised in the psychedelic movement of the mid-Century, and in the pages of Aldous Huxley’s The Doors of Perception, mescaline has been used for many thousands of years by Native Americans.
Mike Jay’s book, Mescaline: A Global History of the First Psychedelic, charts the history of this hallucinogenic alkaloid: from indigenous use and colonial repression through to attempts by Western pharmaceutical companies to commercialize the substance. If published today, Jay’s book may include a thirteenth chapter chronicling the resurgence in interest around classical psychedelics, including the present MindMed study, and apparent interest from other companies such as Journey Colab.
In sum, the book “dwells on the seemingly insurmountable barrier between religious and scientific attempts to understand these substances,” writes David Robertson in a review for Synapsis. This seemingly insurmountable barrier is one that the entire psychedelic ‘space’ grapples with today.
Without delving too deeply into the complex history of mescaline, it’s worth mentioning a few ways in which the drug has been explored in the past century; not least because they stand in stark contrast to the thousands of years of stewardship and religious use by Native Americans. After Austrian chemist Ernst Späth synthesized mescaline in 1919, pharmaceutical giant Merck began marketing mescaline sulfate internationally. This spurred a period of experimentation in many European labs throughout the 1920s and 30s, though such exploration was not particularly fruitful. In its darkest hour, the drug was the subject of abhorrent experimentations at the hands of Nazi physician Kurt Plötner. At the close of the Second World War, Plötner was recruited by the CIA and ultimately worked on MKUltra: another project in which governments sought to harness psychedelics as a tool of power and coercion.
If you can’t find time to digest the entire book, Nature Books and Arts published a short review of Jay’s book, which is open access and worth a read. Additionally, mescaline will be one of the three drugs explored in Michael Pollan’s new book, This Is Your Mind on Plants (forthcoming).
Given a revival of interest in mescaline in recent years, some Native Americans have expressed concern about potential decriminalization and legalization efforts. Blanket decriminalization of psychedelics, for example, could further endanger plants like peyote. The middle ground here may be excluding such plants, and mescaline, from legislative initiatives: the path that Senator Scott Wiener followed when drafting Bill 519.
Other Headlines
We couldn’t possibly cover every news story each week. Below are a selection of other headlines:
- The Chopra Foundation set to partner with MindMed “to educate and build public awareness around the use of psychedelic medicines…”
- Cybin files 12th patent application, covering novel compositions
- Filament Health announces $10m offering and NEO listing application
- MindMed shares Q1 2021 financials
- Mydecine also shared Q1 2021 financials
- FDA approves PharmaTher’s ketamine IND application
- Former Senate majority leader Tom Dasche becomes Special Adviser to Field Trip
- Cybin granted IRB approval for Phase 2 trial in Jamaica
- MindMed finalizes clinical development approach for LSD, with Generalized Anxiety Disorder (GAD) as initial indication
Weekend Reading
fMRI Data Suggest Decreased Brain Network Modularity as Potential Antidepressant Mechanism for Psilocybin Therapy
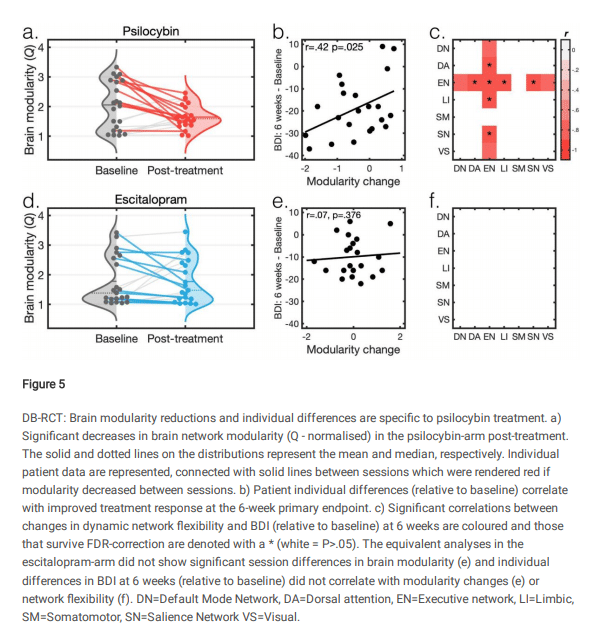
The team at Imperial College London has shared their findings from pre- and post-treatment resting-state fMRI conducted across two psilocybin therapy trials, the latter of which being the recently-published escitalopram vs. psilocybin study published in NEJM.
In a preprint of an article submitted to a Nature journal, the researchers posit that decreased brain network modularity underlies the sustained improvements in depression severity observed in the two studies, concluding:
Consistent efficacy-related functional brain changes correlating with robust and reliable antidepressant effects across two studies suggest a candidate antidepressant mechanism for psilocybin therapy: decreased brain network modularity
Daws, R., Timmerman, C., Giribaldi, B., Sexton, J., Wall, M., Erritzoe, D., … Carhart-Harris, R. (2021). Decreased brain modularity after psilocybin therapy for depression.
Figure 5, below, provides an overview of the findings from the NEJM trial, where psilocybin was pitted against escitalopram.
BBC Documentary: The Psychedelic Drug Trial
BBC 2’s hour-long documentary, The Psychedelic Drug Trial, aired earlier this week. Filmed over 16 month, the programme follows Imperial College London’s escitalopram vs. psilocybin study.
Those in the UK can watch the programme via iPlayer.
Weekly Bulletins
Join our newsletter to have our Weekly Bulletin delivered to your inbox every Friday evening. We summarise the week’s most important developments and share our Weekend Reading suggestions.
Live Updates
Join us on Twitter for the latest news and analysis.
Other Channels
You can also find us on LinkedIn, Instragram, and Facebook.


