Late-Stage Psychedelic Trials
A brief review of the two most mature psychedelic drug development programs
Having completed its second Phase 3 trial of MDMA-AT for PTSD in 2022, MAPS will almost certainly be the first psychedelic drug developer to file a New Drug Application with the FDA in 2023. COMPASS Pathways’ ambitious Phase 3 program, meanwhile, is a milestone for both COMPASS Pathways and psychedelic drug development writ large, representing the most mature clinical investigation of a classical psychedelic. Here, we review both programs.
Part of our Year in Review series
MDMA-Assisted Therapy for PTSD Inches Closer Toward Potential Approval
MAPS Wraps Second Phase 3: Expected NDA Submission Q3 2023 with Potential Approval in 2024
Editor’s note: while it falls outside of the 2022 calendar year, it would be remiss not to mention that MAPS announced in January 2023 that its confirmatory Phase 3 study was “successful”. MAPS PBC confirmed that MDMA-AT reached its pre-specified primary endpoint and key secondary endpoints, similarly to MAPP1. MAPS also reports no serious adverse events (SAEs), meaning no SAEs were observed in the MDMA arms throughout the organisation’s Phase 3 program.
In November, MAPS PBC announced that it had finished its second Phase 3 study of MDMA-Assisted Therapy (MDMA-AT) for PTSD1, MAPP2. Given that MAPS’ Founder and Executive Director Rick Doblin told us back in 2021 that the study was “expected to conclude in November 2022”, the completion was right on time.
This has been a decades-long journey born out of a desire to explore the potential of MDMA-assisted therapy to treat various mental health conditions. Our progress to date would not be possible without the tireless commitment of the investigators, therapists, clinical trial participants and of course MAPS and the thousands of donors who have funded our research to date.
Amy Emerson, CEO, MAPS PBC
MAPP2’s protocol was very similar to MAPP1, the company’s first Phase 3 trial. A key difference, however, was the enrollment of patients with moderate PTSD alongside those suffering a severe diagnosis. MAPP1, meanwhile, only enrolled those with a severe PTSD diagnosis. Demonstrating efficacy in a less severe population would have important implications for the total addressable market of the therapy. The cohort enrolled in the MAPP2 trial was also more diverse than the preceding MAPP1 study, with people of colour representing more than half of patients in the study.
MAPS’ pivotal Phase 3 program was designed under a Special Protocol Assessment (SPA), which the organisation received agreement from the FDA on in 20172. An SPA allows a sponsor, in this case MAPS, to reach agreement with the FDA on the design of a study, including the number of subjects, dosing, endpoints and analysis plans. SPAs represent an effort to ensure that safety and efficacy data produced from clinical trials is acceptable to the FDA for the purposes of market approval.

Now that the data collection phase is complete, analysis and the penning of a study write-up for a peer reviewed journal begins. So too does the immense effort of preparing a New Drug Application (NDA) for FDA review.
A Reminder: MAPP1 Results
MAPS published results from its first Phase 3 trial (MAPP1) in Nature Medicine on May 10th, 2021.
- 88% of participants treated with MDMA-AT had a clinically significant improvement in PTSD symptoms.
- 67% no longer qualified for a PTSD diagnosis.
- No serious adverse events reported in the MDMA group.
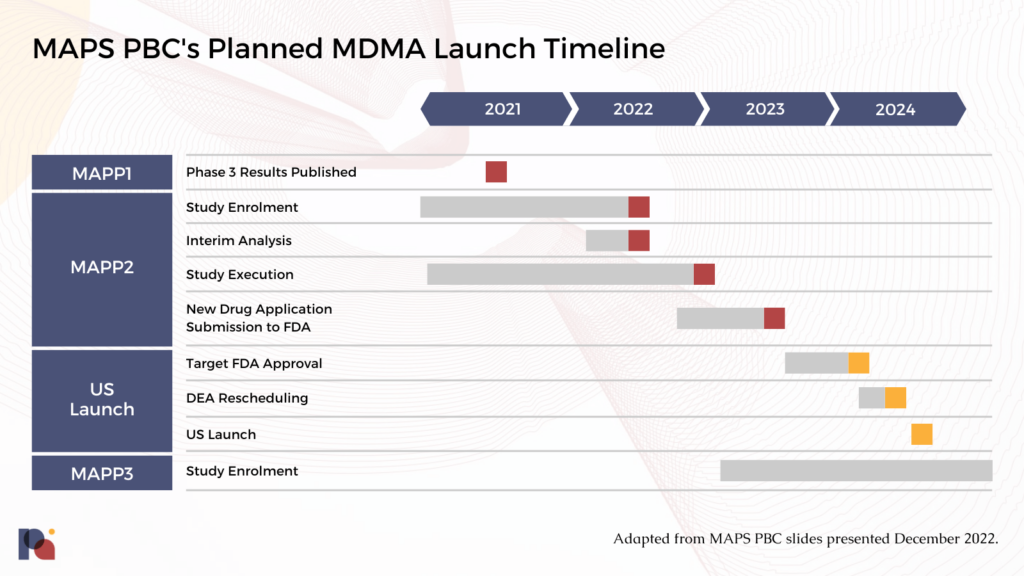
Timelines
The confirmatory Phase 3 trial, which enrolled 121 participants and randomised 104 to MDMA-assisted therapy or placebo with therapy, will be submitted alongside other data in a forthcoming NDA. MAPS PBC told Psychedelic Alpha that it expects to submit an NDA to the FDA in Q3 2023.
Generally, FDA’s review teams aim to take 6 to 10 months to make a decision on whether to approve an NDA, assuming the package is complete. While MDMA-AT will benefit from its Breakthrough Therapy Designation, MDMA is a new molecular entity in the eyes of the FDA, which may entail a slightly longer process (usually tagging on two months to the timeline). It’s also near certain that MDMA-AT will be subject to Risk Evaluation and Mitigation Strategies (REMS), which will take time to devise, negotiate with the FDA, and implement.
If FDA approves MDMA-AT, the DEA rescheduling process could then take up to 90 days3. Aside from this federal rescheduling, states must also reschedule the approved product. Just over half of the fifty U.S. states do this automatically, maintaining parity with federal scheduling decisions. However, that leaves 23 states that don’t reschedule by default. In these cases, MAPS PBC would likely coordinate with state actors to encourage the rescheduling of an approved MDMA drug product.
We should expect any FDA approval of MDMA-AT to take place, at the earliest, in 2024.
This means we should anticipate the following timeline:
If approved, it’s expected that the FDA will require MAPS to undertake paediatric studies of MDMA-AT for PTSD. This would entail testing the safety and efficacy of the treatment in 80 children and adolescents with severe PTSD. 12-17 year olds would be the first patient population eligible for the trial, then 7-12 year olds.
MAPS’ European Phase 3 study, MAPP3, is expected to commence enrolment in Q1 2023. In January 2022, MAPS announced that it had been granted an Innovation Passport under the UK’s Innovative Licensing and Access Pathway (ILAP) scheme. Like the FDA’s Breakthrough Therapy Designation, the ILAP intends to catalyse the drug development process. See our forthcoming Policy section for more.
Regulatory approval of MDMA-assisted therapy would be an enormous milestone for psychedelic medicine. However, beyond achieving approvals, MAPS PBC is wrangling the challenge of ensuring the necessary infrastructure and stakeholder buy-in is available upon commercialisation. Our forthcoming Approvals to Access section covers these considerations in more detail.
COMP360 Psilocybin Therapy for Treatment-Resistant Depression
COMPASS Pathways Takes Its Psilocybin Candidate into Phase 3 Trials for TRD
COMPASS Pathways’ Phase 2b Trial Published in NEJM
Results from London-based COMPASS Pathways’ Phase 2b trial of COMP360 psilocybin therapy for treatment-resistant depression (TRD)4 were published in the New England Journal of Medicine (NEJM) in November (Goodwin et al., 2022). The topline results of the study were originally shared by the company in November 2021.

With this publication, the study becomes the largest published psilocybin trial to date (N=233), knocking NYU’s psilocybin for alcohol use disorder study5 off its perch after just a couple of months.
The trial administered a single dose of COMP360 psilocybin alongside psychological support at one of three doses: 1mg, 10mg or 25mg; where the 1mg dose is thought to be sub-perceptual (and thus approximating a placebo group).
In terms of the headline findings, 37% of those in the 25mg group were responders6 at week 3, which (as the paper points out) “is lower than that described for first-line treatment of major depressive disorder in several large trials of citalopram”, and other conventional antidepressants. Beyond response, remission rates7 at week 3 were 29% in the 25mg group. At week 12, 20% of this group were sustained responders8.
It’s worth remembering, however, that COMP360 psilocybin therapy is not envisaged as a first-line treatment for depression. Indeed, for participants in this Phase 2b trial, it was generally a third, fourth or even fifth-line treatment.
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, a collaborative study operated across 23 psychiatric and 18 primary care sites funded by the NIMH, provides insight into remission rates9 at each conventional (i.e., standard of care) treatment step among a sample of adults with major depressive disorder:

Viewed in this context, the fact that 20% of participants in COMPASS’ Phase 2b trial that received the 25mg dose of COMP360 psilocybin went on to be sustained responders at week 12 takes on some significance.
It’s also worth remembering that we’re only looking at 12 week follow-up data here. Forthcoming results from longer-term follow-up10 with these patients will be critical, as establishing the durability of psilocybin-assisted therapy will determine its clinical and health economic utility.
Head-to-head trials comparing psilocybin therapy to existing protocols, such as CBT, will also be important endeavours. NYU’s Rick Zeifman noted that CBT has been shown to have a 26 % remission rate in TRD patients at a 12-month follow-up, for example.
Achieving sustained remission in 20% of patients is certainly promising, given the treatment-resistant nature of the participants’ diagnoses and when compared to other third- and fourth-line treatments. But, is it the blockbuster result that many had hoped for?
The findings were positive, but not what I could call ‘spectacular’
UCL’s Dr Ravi Das, speaking to Science Media Centre18
Investors appeared to share this mood. Last year, upon publication of the topline results from this trial, the company’s stock took a serious tumble, presumably due to investors’ perception of the results as lacklustre, especially when coupled with the incidence of adverse events.
On that topic, we discussed and contextualised adverse events in our deconstruction of the initial topline results, but since this peer-reviewed publication a number of experts have come to the fore to discuss the matter once again.
Natalie Gukasyan started an interesting discussion on Twitter around how adverse events are defined in the context of psychedelics, and how we can understand their significance in a relatively small sample of people with TRD. Gukasyan supposed that higher rates of suicidal ideation, for example, might be reflective of the desperation seen in patients who didn’t respond to the treatment, especially given the amount of hype around psilocybin for depression. As Johns Hopkins’ Sandeep Nayak notes, supplemental data showed that the 3 patients that displayed suicidal behaviour in the 25 mg group were all non-responders, supporting this thesis.
We were able to glean some additional information from this publication. Notably, it helped elucidate what COMPASS means by “psychological support”, a phrase that the company conspicuously prefers over “therapy”. Some have speculated that COMPASS will seek to achieve reduced labour intensity and increased standardisation in its protocol vs., say, MAPS’ MDMA-assisted therapy for PTSD which presently features two therapists engaging with patients at each session with a high degree of latitude afforded to these facilitators. We discuss this in further detail in a later section on Therapeutic Alliance.
The publication delineates the number of sessions and the therapist:patient ratio at each. At least three preparatory meetings between one therapist and the patient take place prior to the psilocybin session. During the 6-8 hour psilocybin session, the lead therapist11 is accompanied by a second therapist. After the psilocybin session, participants receive two integration sessions with the same two therapists. Here, therapists were instructed to avoid actively guiding participants; but rather to remain “open and supportive”.
Specifics aside, this Phase 2b trial leaves us with many questions. Now, the company will attempt to answer some of the most pertinent questions in its ambitious Phase 3 program.
An Ambitious Phase 3 Program
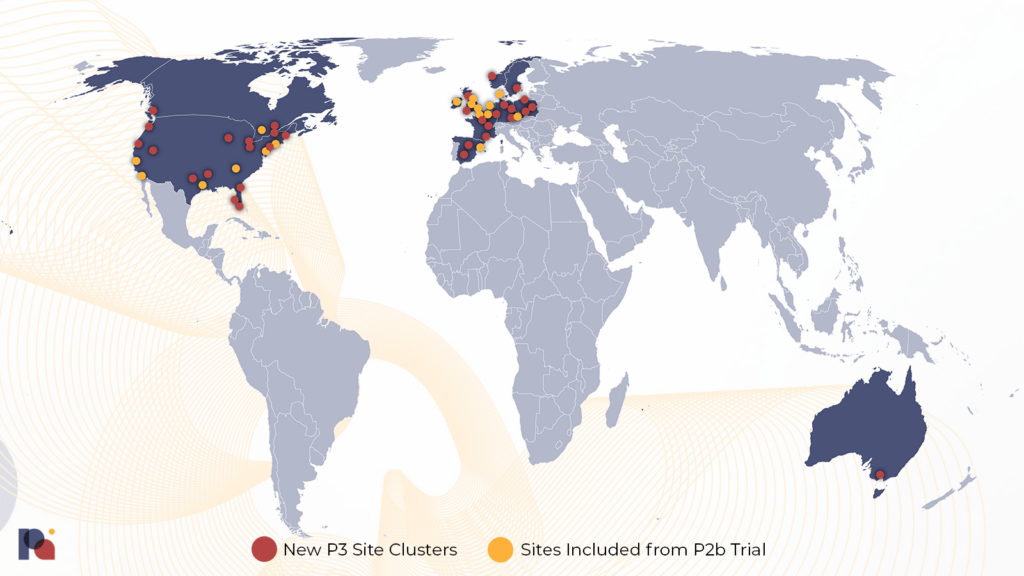
While COMPASS’ Phase 2b trial is the largest published psilocybin trial to date, its Phase 3 program expects to enrol nearly 950 patients across two concurrent pivotal trials. These trials will take place at around 150 sites in fourteen countries.
The two pivotal trials have been designed to answer four key questions pertaining to the safety and efficacy of COMP360:
As shown by the randomisation ratios, COMPASS is most confident in its 25mg dose; unsurprising given their existing evidence base and the academic trials that preceded them.
In terms of psychological support, COMPASS Pathways told Psychedelic Alpha that every patient enrolled in these two studies, “will be assigned a lead therapist and will meet with them for preparation and integration sessions”, who will then be present during the psilocybin session. This 1:1 patient:therapist ratio during the drug administration portion is reminiscent of the company’s Phase 1 study (Rucker et al., 2022), though COMPASS explained that, “an assisting therapist will be available to support as needed.”
Patients will receive 2 preparation sessions before their initial psilocybin session, and 2 integration sessions after each psilocybin session, “which is comparable to our Phase 2b study”, a representative confirmed15.
As explained in the table above, we might expect to see topline data from these studies at the end of 2024 and mid-2025, respectively.
The trial was expected to commence by the end of 2022 (COMP 005 is registered).
Commercial Considerations
Beyond clinical questions, COMPASS Pathways will also need to engage with commercial considerations; especially in light of the anticipated cost of psychedelic-assisted therapies (PATs).
One way the company might look to reduce costs is by reducing the patient:therapist ratio from 2:1 towards 1:1, or even group administration. As aforementioned, it has begun that path in its Phase 3 program, which will see 1:1 ratios, with an assisting therapist available if needed.
However, the company might achieve significant cost savings by being able to whittle out patients who might not respond to its COMP360 psilocybin therapy: ideally, before the first psilocybin administration session16. This appears to be emerging as a clear focus for COMPASS, which has indicated efforts on this front including the employment of artificial intelligence.
We discussed some of the more tech-enabled ways in which COMPASS might seek to achieve these goals in an article for Lucid News17:
So far, we have focused on the key clinical questions that the company’s Phase 3 program will seek to address. But, given the costly nature of psychedelic-assisted therapies, commercial questions abound for companies like COMPASS.
Some companies might attempt to reduce the cost of their protocols by reducing the amount of therapy, or even attempting to replace preparatory and/or integration sessions with a digital therapeutic, for example. They might also aim to reduce the number of therapists involved in the delivery of the protocol, or trial group therapy. While COMPASS often uses the word “therapist”, singular, in its public-facing communications, its published trial shows that two therapists are present at dosing and integration sessions. The company might also develop shorter-acting or non-hallucinogenic psychedelics (or, to use an increasingly common descriptor, “psychoplastogens”).
Not all of these avenues are open to COMPASS’ COMP360 program—which is positively psychedelic—but there are still a bevy of cost-optimising measures available. Not least of these is the embrace of technology.
Today, COMPASS Pathways is keen to position “digital tools” as one of the core pillars of its COMP360 psilocybin therapy, touting a suite of painfully punny tools like its “COMPanion” online therapist platform and “myPathfinder” patient support app. Like some of its peers, COMPASS is presumably hoping that—aside from augmenting the safety and efficacy of its therapy—these tools might also cut costs at varying stages of the patient journey.
To illustrate this point, let’s look at two examples of how this might play out at very different ends of the patient journey.
Theoretically, one of the most propitious ways to boost efficacy and slim down costs is to develop a method of weeding out responders from non-responders as early as possible. In this vein, COMPASS was keen to showcase “Chanterelle”, its program that seeks to apply natural language processing (NLP) in pursuit of this hefty endeavour. The program slurps up qualitative and quantitative data and spits out a prediction of how likely a participant is to be a responder.
According to data shown during the Capital Markets Day (and in a preprint publication), Chanterelle is able to identify responders with a great degree of accuracy, but—and this is crucial—only after the first dose of COMP360 has been administered. Given that the vast majority of costs associated with the single-dose version of this therapy have been spent by that point, it’s akin to Booking.com predicting that you will enjoy your vacation only after you’ve picked up your complimentary coconut water at checkin-in, tested out the firmness of the mattress, and dipped your toes in the pool.
Of course, COMPASS will look to bring forward the predictive window of Chanterelle, which might be expected to only improve as the sample size increases. Might the AI-based tool also be able to predict treatment-emergent serious adverse events, too?
At the other end of the patient journey, companies like COMPASS have teased their plans to use technology on a longer-term basis to identify people who may be slipping into relapse through the analysis of digital biomarkers, for example.
As alluded to earlier, COMPASS might hope that multiple administrations of its COMP360 psilocybin will boost the efficacy of its therapy, or its durability. Indeed, on the final page of the company’s latest presentation, COMP360 is envisioned as an “episodic” treatment, with 1-3 administrations provided over six months.
This might make more commercial sense for COMPASS (as Goldman Sachs analysts crudely asked in a much-memed report four years ago, “Is curing patients a sustainable business model?”), though it will be a careful balance between demonstrating the cost-effectiveness of the therapy and achieving a sustainable business model.
Not everyone is excited about these technologies, though. In a way reminiscent of the chanterelle mushroom’s fruity aroma and peppery taste: some are sweet to the prospect of augmenting psychedelic therapy with technology, while others are concerned by the quantity and sensitivity of data collection that these technologies rely on.
For this Year in Review report, we asked COMPASS about the commercial and clinical value of attempts to predict responders (and, non-responders). The company told us that predicting who is likely to respond, “could help to personalise approaches”, adding that its Phase 2b dataset puts them in, “a unique position”. The company highlighted its effort to develop digital markers from voice, which it hopes will enable it to “stratify the population and predict outcomes.” This is achieved using anonymised conversations between patients and their therapist which, “provides signals that may help determine patient outcome post treatment, identify patients that may better respond and deliver on the promise of personalised, predictive, preventative care.”
It will be interesting to see the utility of such predictive methods in the coming years.
Next, in our Approvals to Access section, we look more broadly at the challenges psychedelic drug developers will face if and when they engage in commercialisation efforts.
Part of our Year in Review series
This content is part of our 2022 Year in Review, which looks back at the past year through commentary and analysis, interviews and guest contributions.
Receive New Sections in Your Inbox
To receive future sections of the Review in your inbox, join our newsletter…
- As we noted on Twitter a week prior, the study was marked as completed with an end date of November 2nd, when the final participant completed their final session. The database was locked and finalised on November 22nd.
- See the letter from the FDA, detailing the SPA agreement, for more detail.
- “Normally, when a drug seeking FDA approval is approved and subsequently recommended for scheduling in Schedules II–V by the Secretary of Health and Human Services (“HHS”), the DEA has ninety days from the later of those two dates to issue what is called an interim final rule, placing the drug on a schedule according to federal scheduling criteria.” (Ali et al., 2022).
- A TRD diagnosis is generally appropriate where a patient has not demonstrated adequate response or remission following two or more treatments.
- We published a deep dive into said study in September.
- Where response is defined as ≥50% decrease from baseline to week 3 in the MADRS total score.
- Where remission is defined as MADRS total score ≤10 at week 3.
- Where sustained response is defined as week 3 response maintained through week 12. This figure appears to be down from the one circulated in topline results and touted by management, which was 24.1%. The reason, it seems, is because COMPASS decided not to go with the definition of “sustained response that is consistent with other TRD studies” when publishing the trial in a peer reviewed journal, instead opting to use the protocol-defined definition. As Psychedelic Alpha’s Medical Advisor Michael Haichin pointed out on Twitter, we don’t know whether this finding reached significance.
- A score of ≤5 on the Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR) defined remission. Fava et al. (2003) elucidate the STAR*D study’s methodology and rationale further.
- Long Term Follow Up Study to COMP 001 And COMP 003 Trials (P-TRD LTFU) (NCT04519957)
- i.e., the therapist that engaged with the patient during the preparatory sessions.
- While many had written off the 10mg dose following lacklustre Phase 2b results, a study published on December 28th (von Rotz et al., 2022) found that a single dose of 0.215mg/kg body weight (around a 16mg dose for a 70 kg patient) alongside psychological support significantly reduced depressive symptoms compared to placebo for up to two weeks. The trial used synthetic psilocybin obtained from COMPASS Pathways.
- Both trials will also include a longer-term follow-up study.
- Once COMP 005 is complete, it’s likely that some of these sites will pivot to support the larger COMP 006 trial.
- We also asked COMPASS if they had any plans to trial COMP360 psilocybin therapy without psychological support. “No studies are planned”, they told us.
- Or, in a multiple administration paradigm, as early as possible following the first psilocybin session.
- COMPASS’ Published Results Take its Psilocybin into Final Phase of Clinical Trials, published November 16, 2022 in Lucid News. We also discussed this in an October Bulletin.
- Das takes issue with the uneven proportions of severely depressed people in different arms of the experiment, noting that there were “significantly fewer severely depressed people in the apparent ‘effective’ (25mg) dose group”, which is not addressed in the paper. Dr. Matthew Johnson dismissed the critique, suggesting it’s not significant.