Psychedelic Policy & Law in 2022
Psychedelic drug policy reform initiatives continued to accelerate in 2022, with North America remaining the most active locale for such efforts. Given the fast-paced nature of such changes, this section briefly reviews a number of key initiatives and themes across the U.S., Canada and Europe.
Part of our Year in Review series
The United States
In December 2022, an article published in JAMA projected that a majority of states will legalise psychedelics by around 2034-2037. While the model used by the researchers placed a lot of credence in the idea that psychedelic drug policy reform will follow a similar temporal and geographic trajectory as marijuana reforms did, it’s nonetheless reflective of a broader feeling that psychedelic drug policy reform is predestined; at least in a majority of the fifty states.
As we noted in a November Bulletin, the state-by-state medical and recreational marijuana timeline does demonstrate some translational utility: California, Oregon and DC, for example, were among the first states to pass medical marijuana legislation in the mid-to-late 1990s. Today, they’re hotbeds of psychedelic drug policy reform initiatives; and successes.
Here, we look at two of the most substantive state-level initiatives that emerged or progressed in 2022, before turning to nascent efforts at the federal level. We then review some themes in psychedelic drug policy reform as well as other legal matters such as litigation and increasing scrutiny of ketamine clinics.
Colorado’s Natural Medicine Health Act Wins Voter Approval
In November 2022, Colorado became the second state to vote for the implementation of a legal psilocybin services program when voters endorsed Proposition 122, the Natural Medicine Health Act (NMHA). Arguably the most substantive psychedelic policy reform measure passed in the U.S. to date, the NMHA creates a state-regulated supervised psilocybin therapy program that should be ready for 2024, and decriminalises a number of psychedelics.
It was a tense run-up to the elections for proponents and opponents of the Proposition alike, with a poll conducted in the last week of October suggesting a very close call. And close it was:
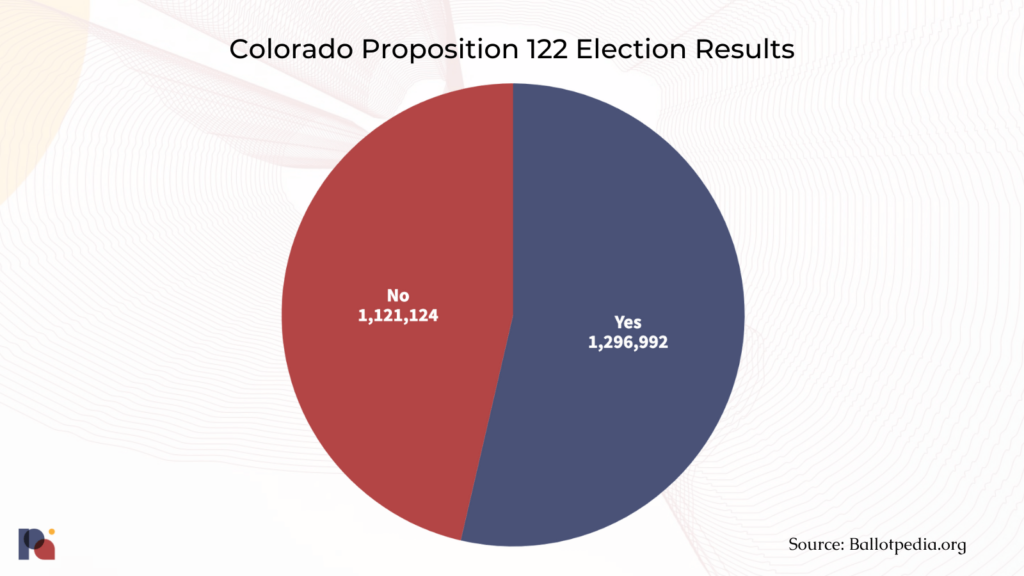
Now, NMHA’s advisory board will stew over the nuts and bolts of the program’s implementation for 18 months.
Oregon’s Psilocybin Services Face a Challenging Start
Oregon’s efforts to establish a regulated Psilocybin Services program by the beginning of 2023 reached a crescendo in 2022, as the realities of the undertaking became apparent to insiders and observers alike.
The most basic of these realities is the ability of Oregon Psilocybin Services (OPS) – the dedicated section housed within the Oregon Health Authority (OHA) to oversee the implementation of the psilocybin services program endorsed by voters in Measure 109 – to remain financially solvent. Once psilocybin services are up-and-running in 2023, the section appeared to have expected to sustain itself via a fee-based budget (i.e., license fees paid by service providers and associated taxes on psilocybin).
But, late in 2022, OPS felt compelled to request additional state general funds (around $6.6 million) to sustain its work through 2023, bridging an anticipated shortfall in funding. Given that the licensing fees are already substantial ($10,000 per year for service centres, manufacturers, and testing labs; $2,000 per year for facilitators), OPS presumably felt that it was not possible to increase them further.
OPS told Psychedelic Alpha that, for the 2023-25 biennium, it has estimated the following number of licensees:
- Facilitators: 750
- Service Centers: 28
- Manufacturers: 26
- Testing Labs: 13
Even if these lofty targets are reached (all signs point to the contrary, at present), it still leaves OPS in a precarious situation and a present shortfall.
To be sure, it’s standard procedure for state agencies to request funds from state governments, with Oregon Health Authority reporting around 50 policy packages in its 2023-25 requests. Nevertheless, it will be interesting to see if and when OPS becomes self-sustaining in the future; as the original Measure had suggested to voters.
New efforts to implement Oregon-esque regulated access programs will likely seek to learn from the Beaver state’s self-imposed restrictions. Colorado, for example, will allow its program’s funds to come from a broader range of sources, including donations and grants1.
Another political reality was rural Oregon’s distaste for psilocybin services, which manifested via an Opt-Out option baked into Measure 109, which allows for local jurisdictions to opt-out of allowing psilocybin services within their borders.
During the November 8, 2022 elections, many Oregonians used this option, with large swathes of Oregon’s landmass turning red on our dedicated Opt-Out Tracker produced in collaboration with Emerge Law Group, Calyx Law, and Healing Advocacy Fund.
Circles represent cities, conforming to the same color-coding as counties.
But, as we explained prior to the elections2, when viewed through the lens of population density the situation is less concerning for proponents of psilocybin services in Oregon.
Take the five most populous counties in Oregon, for example. At the time of our reporting in late August, just two of them put opt-out ordinances on the ballot:
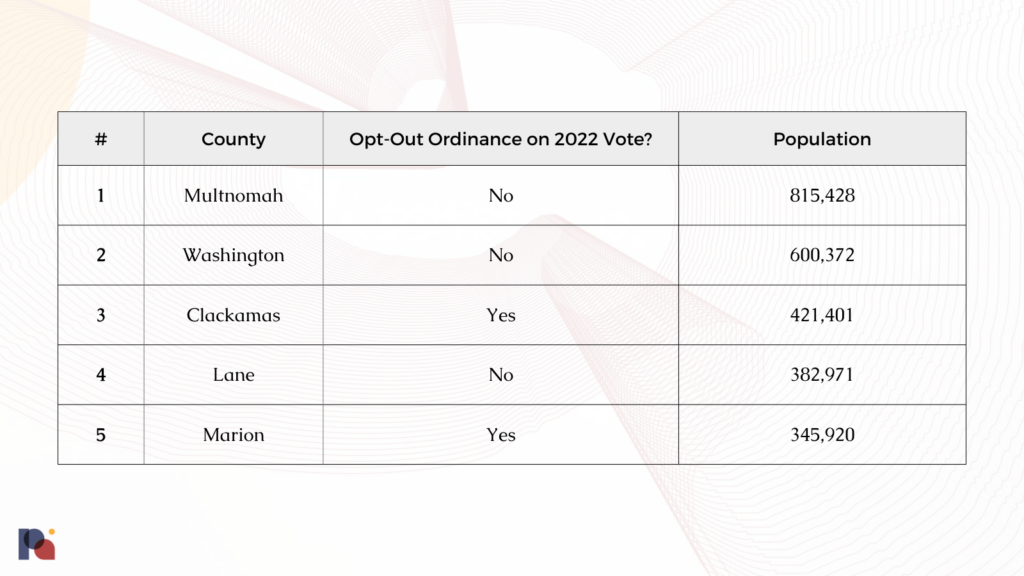
Indeed, both Clackamas and Marion opted-out in the November elections.
With a combined population of around 2.5 million, these five counties alone represent just over 60% of Oregonians. The seven Eastern-most counties – all of which are staunchly Republican, rural, and have low population densities – all voted to opt-out. But, despite representing around a third of the landmass of Oregon, they represent a combined population of around 175,000 people: just over a fifth of Multnomah.
As such, our map is visually quite misleading in showing such large swathes of red, or counties and cities that ‘Opted-Out’ in November 2022. If we adjusted the size of counties on the map by their population, the picture would be much different.
This point was picked up by The Oregonian’s coverage, which noted:
“A map from Psychedelic Alpha shows where opt-outs passed. While it shows nearly two-thirds of the state will not allow psilocybin centers and manufacturing, the places where the majority of Oregon’s population live, the Portland metro area and the majority of the Willamette Valley, are all opted-in.”
It’s even more misleading when you take into account the fact that city land is subject to city regulation: it is only unincorporated land outside of city limits that is subject to county control. As such, cities within opt-out counties could choose to remain opted in, and become ‘safe haven’ cities. These are represented as green blobs amidst red counties on the map.
The population of these cities is often substantial, meaning that even in counties like Clackamas and Marion, the most populated cities like Portland and Salem, respectively, are “safe haven” cities for Oregon Psilocybin Services (i.e., they’re not opted-out).
Such is the importance of delving deeper into the data.
***
As we noted in the days after the elections, there are also a few other points to keep in mind:
- Not all of these opt-outs are permanent; some are temporary moratoriums.
- Public education and engagement appears to work: voters in Deschutes County convincingly rejected the opt-out ordinance. Deschutes was home to one of a handful of campaign groups formed specifically to oppose opt-outs. On this point Sam Chapman, Executive Director of the Healing Advocacy Fund, told Psychedelic Alpha that this shows that “when people hear about the potential of psilocybin therapy from their own community members, they understand why it’s important”.
- It’s important to appreciate the equity and access implications of the new geography of psilocybin services in Oregon.
If Oregon’s psilocybin program proves to be a success, there’s no reason that wider adoption won’t emerge among the presently ‘opted-out’ counties. Fluence’s Victor Cabral told us that, “the hope is that over time, successful implementation and improvement of the Measure 109 program in counties that opt-in will lead to wider adoption across the state.” Chapman agreed, predicting that “as the therapy gets implemented in Oregon communities in 2023 and beyond, voters will continue to learn about how it can help address our state’s mental health crisis, and over time access will only continue to expand.”
Beyond these more existential threats to OPS and the local contours of psilocybin services availability, there are a bevy of logistical issues that would-be psilocybin service centres and other operators are facing.
Harris Bricken’s Vince Sliwoski has been covering these challenges via the firm’s Psychedelics Law Blog, and identifies his ‘top five’ challenges as:
- “The lack of a retail sales or an off-site use allowance, to supplement the on-site, supported adult use model
- The high cost of licensing for facilitators in particular (up to $8K for certification, plus $2K in OHA fees annually)
- The length of administration sessions, which can be six hours minimum at the highest doses (and not reimbursable by insurance)
- The decision by 137 towns and counties to opt out of psilocybin services
- Intractable issues caused by federal prohibition, especially around taxation and lack of banking”
These more mundane issues, which also include zoning and land use rules, will likely further slow or stunt the establishment of psilocybin services in Oregon.
Federal Psychedelic Drug Policy Reform Efforts Emerge
To date, many psychedelic drug policy reform efforts have taken place at the local or state level. However, 2022 saw the emergence of a number of efforts pushing for psychedelic policy reform at the federal level, with varying degrees of coordination. In brief, these include:
- Federal Task Force: In May, a letter from Assistant Secretary of Health and Human Services for Mental Health and Substance Use Miriam E. Delphin-Rittmon to Representative Madeleine Dean explained that SAMHSA is, “exploring the prospect of establishing a Federal Task Force to monitor and address numerous complex issues associated with emerging substances”, with reference to psilocybin and MDMA. Psychedelic Alpha understands that the establishment of such a Task Force is underway, with the involvement of groups like Reason for Hope and The Veterans Mental Health Coalition (VMHC).
- Breakthrough Therapies Act: Introduced by Cory Booker and Rand Paul, this bipartisan effort would reduce barriers to psychedelic research and access3. We provided further details in our December analysis of the act.
- NDAA amendments: In perhaps the most bipartisan pairing of representatives possible4, Dan Crenshaw and Alexandria Ocasio-Cortez forwarded amendments to the National Defense Authorization Act (NDAA) for 2023, which would modestly reduce federal restrictions on certain psychedelic research. The House of Representatives adopted the amendments, which were likely more of a political win than a substantive one. Our commentary from July provides more colour.
- The Congressional Psychedelics Advancing Clinical Treatments (PACT) Caucus: with involvement from a wide array of groups, this new congressional caucus has been celebrated by the likes of MAPS. Its goals include increasing awareness of PATs among members of congress and their staffs, supporting increased federal funding for psychedelic science, and examining regulatory impediments to psychedelic research. The Caucus will not offer recommendations on the decrim., legalisation, or re/de-scheduling of psychedelics.
Other Themes in U.S. Psychedelic Policy and Law in 2022
Local Decriminalisation Efforts Continue
- As you can see from the non-exhaustive timeline at the very bottom of this webpage, a number of decriminalisation efforts made headway in 2022.
- Take California, for example. While a state-wide psychedelic decrim. effort was resuscitated in late 2022, local decriminalisation efforts continued in the Golden State, with San Francisco passing something akin to decriminalisation of psychedelics (though, not exactly) and the city of Berkeley looking to decriminalise a number of psychedelics (including LSD) via a resolution that passed the Berkeley Health Commission in late 2022.
At-Home Ketamine Comes Under Scrutiny
- As a COVID-era waiver to the Ryan Haight Act allowing remote prescribing of scheduled substances looks set to sunset in 2023, where does this leave ketamine telehealth companies? When viewed in the context of a growing number of unfavourable media reports of these at-home ketamine offerings, some companies are bracing for impact.
- As we covered in May 2022, the fate of San Francisco-based mental health startup, Cerebral, may be a canary in the coalmine5. The company, which became known for prescribing Adderall (a Schedule II substance) without in-person consultations, is under investigation by the U.S. Department of Justice for potential violations of the Controlled Substances Act.
Psychedelics-Related Litigation Ramps Up
In 2022, we saw a number of psychedelics-related litigation headlines. While it’s still a small crop, it’s interesting to see a variety of cases emerge, from inter-company complaints right through to challenges to the DEA. Here are a handful of examples:
- DEA Ditches Plan to Schedule 5 Tryptamines: In the Summer of 2022, the DEA scrapped a proposal to place five tryptamines on Schedule I after a group of researchers, psychedelic startups and lawyers challenged the agency. We sifted through around two hundred documents related to the case to produce our most in-depth Special Report of 2022, Inside the Challenge to DEA’s Proposed Scheduling of 5 Psychedelic Tryptamines.
- DOI and DOC Escape Scheduling: Later in 2022, the DEA backpedalled on another attempt to schedule psychedelics. While both instances of the DEA bowing to public pressure and legal challenges are promising, many expect the agency to return with fresh plans to schedule such substances.
- Terran Biosciences files lawsuit alleging that COMPASS “milked” researcher for trade secrets before “secretly” filing patent on same technology: This complaint, which is understood to constitute the first trade secret lawsuit in the psychedelics space, was filed on the 5th of August, 2022. We covered it in an August Bulletin.
- Two Post-Grant Reviews Denied: In June, COMPASS Pathways survived two challenges to its Polymorph A patents, which are understood to cover its crystalline forms of psilocybin including COMP360. The challenges were brought by Freedom to Operate, a non-profit group founded by Carey Turnbull dedicated to “protecting psychedelic science and medical development for public benefit”. At the time we described this as, “an unreserved win for COMPASS”, explaining that the Patent Trial and Appeal Board (PTAB) had sided with the company on both of the claim constructions, finding each of FTO’s invalidity arguments unsupported and unpersuasive. Requests for rehearing and further review, filed in July and August, remain pending.
- AIMS v. DEA: see Kathryn Tucker’s description of events. Though, as with all contributions to this Review series, do note that new developments may have emerged since the time the contribution was authored.
The Emerging Role of the VA
- Given the politically and culturally salient nature of veterans in the U.S., it’s unsurprising that many policy reform and advocacy efforts are foregrounding the plight of many in this community. In October, the Department of Veterans Affairs published an Evidence Brief, Psychedelic Medications for Mental Health and Substance Use Disorders. “Evidence is therefore very preliminary and several critical gaps need to be addressed by future research”, the report concludes.
Canada
Canada has been a hotbed for psychedelic business and advocacy for a number of years, from its Special Access Program amendments through to successful localised efforts in the (psychedelic) drug policy reform arena. Here, we look at a handful of developments from 2022.
Special Access Program (SAP) Amendments Provides Very Modest Access to PATs
Near the end of December 2020, Canada’s leading health agency, Health Canada, notified the general public of its intent to pursue regulatory amendments which would restore access to “restricted drugs”6 through the agency’s Special Access Program (SAP). Over the course of a two-month public consultation period, Health Canada received 392 responses from individuals and organisations. Interestingly, expanded access to psychedelics was a leading theme in responses gathered by Health Canada. As the agency described:
“80% of all respondents associated Health Canada’s proposal with increasing access to psychedelic restricted drugs (e.g. MDMA, psilocybin, LSD, DMT), often for the treatment of various conditions, most notably mental health disorders.”
On January 5, 2022, Health Canada’s amendments were announced, allowing the Minister of Health to authorise, “the sale of an unapproved drug from a manufacturer to a practitioner for the emergency treatment of a patient under certain circumstances.” However, SAP requests for psychedelics are limited to only psilocybin and MDMA (Pazdan & Goldgrub, 2022).
In its Gazette notice, Health Canada noted how many public consultation respondents erroneously believed that these regulatory changes would allow for a, “guaranteed access to psychedelic restricted drugs.” In light of these frequent misinterpretations, the agency emphasised that its amendments would not support such widespread access to restricted drugs, nor should they be interpreted as an indication of Health Canada’s desire to move towards broader decriminalisation or legalisation.
Instead, access requests are to be submitted on behalf of qualifying patients by eligible practitioners through the SAP. Submitted requests will be considered “on a case-by-case basis” and, in doing so, the agency will take into account the existing body of clinical evidence that supports the use of the drug(s) for a patient’s condition. Interestingly, Health Canada described how requests “are normally only considered when positive results of Phase II or Phase III clinical trials are already available.”
Furthermore, as Pazdan & Goldgrub (2022) explain:
“SAP requests, however, will only be granted for patients with a ‘serious or life-threatening condition’ in cases where other conventional treatments have failed, are unsuitable for the patient, or are not available in Canada.”
Prior to Health Canada’s recent SAP amendments, patients hoping to access psychedelics required the Minister of Health to grant an exemption to the Controlled Drugs and Substances Act (CDSA) under Section 56 of the act. While dozens of patients have successfully gained access to psilocybin using the section 56 exemption pathway, wait times can be notoriously long. Furthermore, patients granted these exemptions are required to source their own drugs.
Conversely, patients who obtain access to psilocybin or MDMA using the recently-amended SAP are not required to source their own supply. Instead, Health Canada-approved dealers provide their manufactured drugs to the patient’s health care provider.
While the new process was widely viewed as a step in the right direction, shortly after the amendments came into effect applicants, many of whom had sought access exemptions through the section 56 pathway, began to have their applications denied by Health Canada.
In one such case, Health Canada informed a patient that they “had not exhausted all possible legal routes available” to obtain access to psilocybin, and as a result were not being approved for an exemption under section 56 of the CDSA. In fact, as a CBC article published on February 28, 2022 described, zero section 56 exemptions had been granted since psilocybin was added to the SAP list earlier in 2022.
Thus, it appears that in the eyes of Health Canada patients seeking medical access to certain psychedelics should first apply through the amended SAP, and rely on section 56 of the CDSA as a secondary option.
However, these rejections applied not only to patients seeking medical access, but also to health care providers who sought section 56 exemptions to access psilocybin for training reasons. In fact, near the beginning of 2022, Health Canada rejected the applications of roughly 80 Canadian healthcare professionals. In response, Health Canada suggested that practitioners interested in training should do so by participating in an approved clinical trial.
These rejections and limits on access to psychedelics prompted seven patients and one practitioner to file a lawsuit against the Canadian Government and Minister of Health. The plaintiffs, with support from TheraPsil, are “arguing for a constitutional right to individualised psilocybin-assisted therapy” (Pazdan & Goldgrub, 2022).
The charter challenge cites a number of important deficiencies (prohibitions) that unjustly limit existing options available to patients and providers who wish to access psilocybin. Accordingly, the group claims that the prohibitions referenced in their challenge violate section 7 of the Canadian Charter of Rights and Freedoms, which guarantees “the right to life, liberty and security of the person”.
As Pazdan & Goldgrub (2022) describe in their review of Canadian psychedelic law, the challenge does not appear to be without strong precedent:
“The patients’ argument is a strong one, clearly echoing that of the successful 2000 Parker case. In Parker, the Ontario Court of Appeal held that the law broadly prohibiting access to medical cannabis violated the epileptic plaintiff’s Charter rights. Notably, the court regarded the possibility of the plaintiff accessing medical cannabis by means of s. 56 exemption as insufficient to save the government’s legal stance. As a remedy, the court mandated that the federal government create Canada’s first regulated, individualized medical cannabis regime within a year.”
As we move further into 2023, it will be interesting to see how this Charter challenge unfolds, and whether it might have broad impacts on medical access to psilocybin in Canada.
While the amended SAP certainly failed to meet the needs or expectations of many, on March 21, 2022, Health Canada authorised Dr. Valorie Masuda to treat six patients for their end-of-life distress using psilocybin. Subsequently, in May 2022, Numinus Wellness and Braxia Scientific both announced that they had received SAP approval from Health Canada to treat patients suffering from depressive disorders using psilocybin.
More positive news emerged near the end of the year, when TheraPsil announced that two physicians had successfully billed for and were paid by the province of Quebec “after completing psilocybin-assisted psychotherapy for a patient with legal access”, making the province of Quebec the first to cover the costs of psilocybin-assisted therapy.
Alberta Becomes the First Province to Regulate PATs
On October 5, 2022 the government of Alberta announced that it would be amending province’s Mental Health Services Protection Regulation to, “introduce new requirements for psychedelic assisted therapy.” The amendments, which came into effect in January 2023, made the province the first to regulate psychedelic-assisted therapies in Canada
Pursuant to the new regulations, only a “psychiatrist or a physician in consultation with a psychiatrist” will be permitted to prescribe psychedelics to patients. Furthermore, service providers (medical facilities) who wish to deliver psychedelic therapies must, “apply and hold a licence under the Mental Health Services Protection Act”, in addition to appointing “a psychiatrist as a medical director” who is charged with overseeing “all clinically related aspects of the licensed services.”
Psychedelic therapies in Alberta must take place only at a medical facility or clinic, except in cases of end-of-life care. Patients are also not permitted to self-administer the drugs: psychedelics can only be administered by an eligible health professional, who is then required to “monitor, treat and care for the patients while in an altered state of consciousness.” These care providers include psychiatrists, clinical psychologists, and other authorised health care professionals such as occupational therapists, nurses, and social workers.
While a number of pro-psychedelics groups heralded the announcement as a success, it’s not so simple. These new regulations could be used as a way to bear down on unlicensed providers, as opposed to a signal that the province intends to institute pre-approval access to PATs in any meaningful way. It’s important to remember that the regulation of drugs and controlled substances falls under the remit of the Government of Canada, not provincial governments.
While these changes demonstrate an awareness of psychedelic therapy, and some level of expectation that PAT is coming down the pike, it’s unlikely that they represent any meaningful increase in the accessibility of psychedelic therapy for now.
BC Decriminalises Possession of Certain Amounts of Controlled Substances, Including MDMA
In November 2021, the Canadian province of British Columbia (BC) submitted an “exemption request” under Section 56(1) of the Canadian Controlled Drugs and Substances Act (CDSA) to the country’s federal health agency. In its submission, the province described how the “criminalization of simple possession remains a significant impediment to BC’s ability to implement a comprehensive public health response to the illicit drug poisoning crisis.” Accordingly, the province sought to decriminalise personal possession of certain amounts of controlled substances.
On May 31, 2022, representatives from Health Canada announced that BC’s request had been granted, and that the exemption would come into effect later in the new year. As of January 31, 2023, adults in BC (18 years and older) will no longer face criminalization for the possession of less than 2.5g of an opioid, crack and powder cocaine, methamphetamine, or MDMA. Instead, individuals found to be in possession of less than 2.5g of these substances will be “offered information about health and social supports.”
Whac-A-Shroom: Psychedelic Dispensaries Pop Up, Slapped Down, Across Canada
Many Canadians might remember the surge in transient, unlicensed cannabis dispensaries that began to pop up well in advance of the federal government’s move to legalise and regulate the drug for recreational use. While many of these establishments were (repeatedly) shut down by local police action, the unregulated industry proved to be a rather resilient thorn in the side of municipalities and politicians who sought to put an end to illegal dispensaries operating in their jurisdictions.
Now, a few years after licensed cannabis dispensaries were permitted to open their doors across the country, a new group of speciality shops have begun to appear. While not yet as pervasive as their cannabis predecessors, magic mushroom dispensaries are growing in popularity in many of Canada’s most populous cities. Over the course of 2022, cities like Vancouver, Toronto, Ottawa, and Hamilton all saw new brick and mortar establishments open dedicated to selling magic mushroom products, in spite of the drug’s status under Canada’s Controlled Drugs and Substances Act.
Some of these new shops have successfully evaded municipal or police action for periods of time. However others, like the Mushroom Cabinet in Hamilton, have not been so fortunate. In late December it was announced that, just one day after opening its doors, Hamilton’s first dispensary of its kind was shut down by police.
In light of the absence of any federal commitment to psychedelic decriminalisation or legalisation, it will be interesting to see how this unregulated, public-facing industry develops, and how different jurisdictions across the country respond to their presence.
Health Canada Issues Guidance for Psychedelic Clinical Trial Sponsors
Near the end of 2022, Health Canada issued guidance outlining its “expectations regarding the implementation of risk-management measures” by sponsors of clinical trials investigating psychedelics. As we discussed in a twitter thread on the announcement, Health Canada recognized the growing interest in psychedelic-assisted psychotherapy, and due to the potential “psychological and physical risks” trial participants face, the federal agency felt the need to establish best practice guidelines for clinical trials.
In its notice, Health Canada described a number of “risk-management measures” that are to be addressed and included in any clinical trial application. Highlights of these measures include:
- Proper training for therapists on “evidence-informed protocols” for PAT.
- Therapists should be licensed to provide psychotherapy, or, in cases where therapists teams are not regulated, members “should be under the direct supervision of a licensed therapist.”
- “Rapport between the clinical trial participant and the therapist is critical”.
- “During the phase in which the drug is being administered to the participants, there should be a minimum of two therapists present.”
- “The therapists should remain the same through the clinical trial for each patient in order to maintain trust.”
- “Lodging should be available on the day of the administration of the drug in cases where it is preferable to keep participants overnight to help ensure their safety.”
- “Throughout the clinical trial, sponsors are required to report any serious unexpected adverse drug reactions to Health Canada.”
- “Consent for touch should be obtained prior to the administration of the investigational product, as well as in the therapeutic moment.”
- “Any physical contact between the therapist(s) and the participant must be of a non-intimate and non-sexual nature.”
Europe
European policymakers and psychedelic drug policy reform advocates might have got off to a slower start than those ‘across the pond’, but there are promising developments at the Member State and EU-wide levels.
A Member of the European Parliament, Robert Biedroń, told Psychedelic Alpha: (from Poland and representing political group of the Progressive Alliance of Socialists and Democrats):
“In Europe, over 100 million citizens are affected by mental health conditions and substance use disorders, and treatment options for these diseases are often suboptimal. Many members of the European Parliament – including myself – recognize a tremendous potential of psychedelic-assisted therapies in addressing huge unmet needs in those therapeutic areas. This year, we will work with PAREA to establish in the European Parliament an alliance of supportive parliamentarians to raise awareness in the Parliament about those novel treatments and call upon the European Commission and EU member states to already start preparing for their likely approval by the European Medicines Agency in the coming years.”
Tadeusz Hawrot, founder of the Psychedelic Access and Research European Alliance (PAREA) which emerged in 2022, is in an excellent position to provide an update on EU-level discussions regarding psychedelics.
Editor’s note: remember that the remit of this Year in Review is limited to events that happened in 2022, so we (nor our contributors) have not mentioned promising indications from early 2023 in this report, which include a related publication in the Lancet co-authored with European Medicines Agency representatives.
Beyond supranational efforts, individual Member States are looking to better understand, and engage with, the emerging psychedelic medicine landscape. One such country is Czechia (the Czech Republic), which has a fascinating history with psychedelics and related research; especially LSD. Today, there are a number of instances of psychedelics entering into discussions among policymakers in the central European country.
We are grateful to Tereza Dleštíková, Assistant Professor and Researcher at the Police Academy of the Czech Republic in Prague, who has provided us with a brief dispatch covering these developments:
Appendix: A Non-Exhaustive Timeline of Psychedelic Bills and Resolutions in 2022
January 1, 2022
Missouri HB 2429 Filed
On January 1, 2022, Rep. Michael Davis (R) filed HB 2429, which was originally introduced February 18, 2021, as HB 1176. If passed, this bill would expand Missouri’s Right to Try Act to no longer prohibit people with terminal or life-threatening illnesses from using substances such as MDMA, psilocybin mushrooms, LSD, DMT, mescaline or ibogaine with a doctor’s recommendation after exhausting all other approved treatment options, if they qualify as an “investigational drug.” The bill would also reduce penalties statewide for low-level possession of those drugs.
January 10, 2022
Kansas HB 2465 Introduced
On January 10, 2022, Kansas lawmaker Rep. Aaron Coleman (D) introduced HB 2465, aimed at reducing the penalty for individuals cultivating or possessing small quantities of certain controlled substances. This bill comes following a failed effort to pass similar legislation, HB 2288, introduced by the same legislator in February 2021.
January 14, 2022
Vermont HB H.644 Introduced
On January 14, 2022, House Bill H.644 was introduced and referred to the Judiciary Committee. This bill is an act relating to decriminalization of a personal use supply of a regulated drug. The bill proposes to change the penalties for possession of a personal use supply of drugs from a misdemeanor or low-level felony to a civil offense subject to a $50.00 penalty. A person cited for such an offense may avoid paying the penalty by agreeing to participate in a screening for substance use disorder treatment and related services. The bill would also establish the Drug Use Standards Advisory Board for the purpose of determining the benchmark personal use dosage and the benchmark personal use supply for regulated drugs with a goal of preventing and reducing the criminalization of personal drug use. If the bill is passed, sections 5-14 will take effect on January 1, 2024.
January 18, 2022
Missouri HB 2469 Introduced
On January 18, 2022, with the introduction of HB 2469, Rep. Peter Meredith (D) proposed to decriminalize possession of small quantities of several scheduled substances, including MDMA, LSD, and psilocybin. Passage of this bill would create a three-tiered penalty system for possession of the outlined substances. For example, the penalty for “possession of not more than…one gram of MDMA,…forty units of LSD, [or] twelve grams of psilocybin” would be changed from a class D misdemeanor to “an infraction punishable by a fine not to exceed one hundred dollars or participation in a treatment program…or both.” Possession of more than these outlined quantities would be considered either a class A misdemeanor or a class D felony depending upon the exact amount.
January 24, 2022
Virginia HB 898 Shelved
On January 12, 2022, State Representative Dawn Adams (D), a nurse practitioner, introduced HB 898 which called for the decriminalization of peyote and ibogaine possession in addition to psilocybin and psilocin. If passed, this bill will reduce the penalty for possession of psilocybin, psilocin, ibogaine and peyote for individuals aged 21 and over from a Class 5 felony to a civil offense carrying a maximum $100 fine. Similar to SB 262, HB 898 also provided that “any civil penalties collected” for possession of the named scheduled substances “shall be deposited into the Drug Offender Assessment and Treatment Fund.” This fund would be used by the state assembly to support various treatment programs for individuals struggling with substance abuse. On January 24, 2022, HB 898 was shelved for the legislative session.
January 31, 2022
Virginia SB 262 “Passed By Indefinitely”
On January 11, 2022, SB 262 was introduced by State Senators Ghazala Hashmi (D) and Jennifer Boysko (D) to decriminalize possession of psilocybin and psilocin and referred to the committee on the Judiciary. The bill provides that any person 21 years of age or older who knowingly or intentionally possesses psilocybin or psilocyn shall be punished by a civil penalty of no more than $100 and such civil penalties shall be deposited into the Drug Offender Assessment and Treatment Fund. Under current law, a person who knowingly or intentionally possesses psilocybin or psilocyn is guilty of a Class 5 felony. On January 31, 2022, the Senate Judiciary Committee voted to change the status of the bill to “passed by indefinitely” . The committee may reconsider the legislation at a later meeting but if the committee takes no further action, the bill is dead.
February 1, 2022
Oregon SB 1580 Introduced
SB 1580 would create a task force to investigate and make recommendations about how to address issues related to equity and access to psilocybin service under Oregon’s burgeoning psilocybin services program. The task force is charged with making recommendations about a variety of equity and access topics including how to address barriers keeping people of color and people who are low income from participating in Oregon’s burgeoning psilocybin services program as business owners, facilitators, or clients. If this bill is enacted, the task force would be required to report their findings no later than November 1, 2022.
February 7, 2022
Oklahoma HB 3174 Introduced
Introduced by Rep. Phillips, HB 3174 would create a clear pathway for “academic medical centers” and physicians licensed in Oklahoma to obtain certification to conduct clinical trials using psilocybin. The bill specifies that only veterans aged 18 or older suffering from various forms of mental illness may participate in the clinical trials.
March 1, 2022
Missouri HB 2850 Introduced
On March 1, 2022, HB 2850 was introduced by Rep. Tony Lovasco (R) which proposing to legalize certain “natural medicines” including: ibogaine, psilocybin and psilocin if derived from fungi, DMT and mescaline excluding Lophophora williamsi (peyote) to treat a variety of medical conditions.
March 2, 2022
Rhode Island HB 7715 Introduced
On March 2, 2022, HB7715 was introduced proposing to build upon Rhode Island’s existing marijuana decriminalization policy by decriminalizing possession of psilocybin and buprenorphine. If passed, possession of up to one ounce of “buprenorphine, psilocybin and the substance classified as marijuana” would be exempted from criminal penalties associated with possession of scheduled substances. While “the substance classified as marijuana” is currently exempt from criminal penalties, possession of up to one ounce is considered a civil offense carrying a $150 fine and requiring forfeiture of the substance. However, as currently proposed, possession of up to one ounce of “psilocybin” or “buprenorphine” would not carry any civil penalty under this bill.
March 4, 2022
Rhode Island HB 7896 Introduced
On March 4, 2022, another bill proposing to decriminalize possession of certain drugs, HB 7896, was introduced. If enacted, this bill would decriminalize possession of up to one ounce of any “controlled substance classified in schedules I, II, III, IV, and V, except the substance classified as fentanyl.” Possession of up to one ounce of these controlled substances would result in a $100 fine for a first offense, and up to $300 for subsequent offenses.
March 8, 2022
Maryland HB 1054 & SB 0784 Introduced
On March 8 2022, HB 1054, and SB0784 were introduced which both propose to decriminalize possession of different “de minimis quantities of dangerous controlled substances,” including up to “40 user units of lysergic acid diethylamide (LSD),” and up to “1 gram or 5 tablets of 3,4-Methylenedioxymethamphetamine (MDMA).” If either bill is enacted, possession of “de minimis quantities of dangerous substances” would be considered a civil offense carrying a maximum fine of $100 for a first offense, followed by a $150 maximum fine for a second offense. Third, and all subsequent offenses would result in a fine not to exceed $200. Under current laws, drug possession may carry prison terms and a fine of up to $5,000.
March 8, 2022
Hawaii SB 3160 Approved By State Senate
On January 26, 2022, Senate Bill 3160 was proposed to have the Hawaii Department of Health create a “therapeutic psilocybin working group to examine the medicinal and therapeutic effects of psilocybin and develop a long-term strategic plan to ensure the availability of therapeutic psilocybin or psilocybin-based products that are safe, accessible, and affordable for adults twenty-one years of age or older.” If formed, the therapeutic working group would be tasked with examining: laws and regulations related to the therapeutic use of psilocybin; available medical research pertaining to the safety and efficacy of psilocybin in treating mental health conditions; and the “requirements, specifications, and guidelines for a medical professional to prescribe and provide access to psilocybin to patients in jurisdictions where psilocybin is approved to treat mental health conditions.” On March 8, 2022, SB 3160 was unanimously approved by the Hawaii State Senate and has advanced to the State House for further consideration.
March 16, 2022
Pennsylvania HB 2421 Introduced
On March 16, 2022, HB 2421 (previously HB 1959) was introduced by Rep. Tracy Pennycuick (R) and referred to the Health committee. The Psilocybin Data Act provides for research and clinical studies of psilocybin and psilocybin-assisted therapy. The act provides a framework for research to discover innovative methods to optimize the public health benefits of psilocybin.
March 22, 2022
Utah HB 167 Signed Into Law
On January 17, 2022, House Bill 167 was introduced by Utah State Rep. Brady Brammer (R). This bill proposed the creation of a task force charged with “provid[ing] evidence-based recommendations on any psychotherapy drug that the task force determines may enhance psychotherapy when treating a mental illness.”
On March 22, 2022, Utah Governor Spencer Cox signed 64 bills into law, including HB167, which created the Mental Illness Psychotherapy Drug Task Force. The legislation required a task force to report to the Utah State Legislature’s Health and Human Services Interim Committee about the use of certain scheduled compounds in mental health treatment by October 31, 2022.
March 22, 2022
Hazel Park (MI) Resolution Passed
On March 22, 2022, the city council for the City of Hazel Park passed a resolution to decriminalize “Entheogenic Plants” which include “the full spectrum of plants, fungi, and natural materials and/or other extracted compounds, limited to those containing the following types if compounds: indole amines, tryptamines, phenethylamines; that can benefit psychological and physical wellness, support and enhance religious and spiritual practices, and can reestablish human’s inalienable and direct relationship to nature.” The resolution prohibits the use of city funds or resources to investigate, detain, arrest, or prosecute individuals found planting, cultivating, purchasing, transporting, distributing, engaging in practices with, or possessing Entheogenic Plant. However, the resolution specifically disallows “possessing or distributing these materials in schools, consumption, or usage by minors, driving under the influence of these materials, public disturbance, or commercial sales or manufacturing” of Entheogenic Plants.
March 30, 2022
Georgia HR896 Amended
On March 30, 2022, the House Defense and Veterans Affairs Committee amended the composition of the proposed House Study Committee on Alternative Post-Traumatic Stress Disorder Treatment Resources for Veterans to include an agent of the Georgia Department of Public Health. Following the adoption of this amendment, the House Defense and Veterans Affairs Committee voted to approve the legislation. It will now move to the House Rules Committee for further consideration.
March 31, 2022
Washington SB 5693 Becomes Effective
March 10, 2022, the Washington state legislature sent a state budget bill, SB 5693, to the governor’s desk that included a proposal to direct $200,000 in funding to support a new workgroup to study the possibility of legalizing psilocybin services in the state, including the idea of using current marijuana regulatory systems to track psychedelic mushrooms.
The proposed budget became effective on March 31, 2022. The work group will receive $50,000 of funding for FY 2022 and the remainder for FY 2023. A final report on the group’s findings must be submitted by December 2023.
March 31, 2022
New Hampshire HB1349-FN “Laid On The Table”
On January 5, 2022, HB1349-FN was introduced to the House and referred to the Criminal Justice and Public Safety committee. The act would have decriminalized the possession or use of a certain amount of psilocybin mushrooms by a person 18 years of age or older. On March 31, 2022 the bill was ‘laid on the table.’
April 11, 2022
Oklahoma Senate Committee Votes In Favour Of HB 3414
Sponsored by Rep. Pae, HB 3414 also proposes increasing psilocybin research but provides an alternative approach which would allow anyone over 18 suffering from a list of specified conditions to participate in clinical trials. Additionally, this bill would decriminalize possession of small quantities of “psilocybin- or psilocin-containing fungi or plants,” making possession of less than 1.5 ounces, or approximately 42.5 grams, punishable by a civil penalty of $400 or less.
On March 7, the republican-controlled House of Representatives voted overwhelmingly, 62-30, to pass the legislation, following which the bill moved to the Oklahoma Senate.
On April 11, 2022, the Oklahoma Senate Committee on Health and Human Services voted in favor of passing HB 3414 after amendment. The committee amendment to HB 3414 removed the provisions that would have decriminalized possession of “psilocybin- or psilocin-containing mushrooms.”
April 11, 2022
Aspen (CO) Approves Petition for Proposed Ballot Measure
On April 11, 2022, the city of Aspen approved a petition for a proposed ballot measure, which would make enforcing laws related to the “therapeutic use” of “plant medicines,” such as “ayahuasca, ibogaine, dimethyltryptamine, mescaline, psilocybin or psilocin” the “lowest law enforcement priority in the City of Aspen.” The term “therapeutic use” includes the “possession, storage, planting, cultivating, and transporting of plant medicines” by an adult over the age of 21. The organization behind the proposed measure, Right to Heal, had until October 11, 2022, to submit the required number of signatures to get the measure included on the ballot for the March, 2023 election.
April 19, 2022
Maine SP496 Dies Between Houses
On April 19, 2022, the Maine Senate voted to pass SP496. However, shortly thereafter, the Maine House of Representatives declined to advance the proposal. Following the proposal’s stall in the Maine House, Senator Bailey indicated in an interview with Marijuana Moment that she intends to reintroduce the proposal in the next legislative session or potentially pursue a peoples’ referendum.
May 7, 2022
Connecticut HB 5506 Signed
On May 7, 2022, the Governor signed House Bill 5506 adjusting the state budget for the biennium, effective from July 1, 2022 to June 30, 2023. This state budget bill specifically earmarked funds for psychedelic-assisted therapy programs administering psilocybin and MDMA treatments. Under the pilot program, veterans, retired first responders, and direct care health care worker can be qualified to receive MDMA-assisted or psilocybin-assisted therapy under the supervision of an approved federal Food and Drug Administration treatment site.
May 24, 2022
East Lansing (MI) Resolution Voted Against
On May 24, 2022, the East Lansing City Council voted 3-2 against passing a resolution, which would have made “the investigation and arrest of persons for planting, cultivating, purchasing, transporting, distributing, engaging in practices with, or possession of Entheogenic Plants” among the lowest law enforcement priorities.
May 27, 2022
Maryland SB 0709 Allowed To Take Effect
On April 8, 2022, Maryland lawmakers sent a SB0709 to the governor that would create a state fund to provide “cost-free” access to psychedelics like psilocybin, MDMA and Ketamine for military veterans suffering from post-traumatic stress disorder (PTSD) and traumatic brain injury.
On May 27, 2022, Maryland’s governor Larry Hogan (R) announced that he will allow SB0709 to take effect without his signature. The measure from Sen. Sarah Elfreth (D) passed unanimously through both chambers before being sent to his office.
June 1, 2022
Michigan Ballot Initiative Deferred to 2024
On February 1, 2022, reform activists proposed a ballot initiative that would overhaul Michigan drug laws. If passed, this initiative would decriminalize possession of Schedule 1 and 2 substances. Additionally, the initiative identified psilocybin, psilocin, ibogaine, peyote, and dimethyltryptamine as “Natural Plants and Mushrooms,” which would be legal for anyone over 18 years old to cultivate, possess, use, or gift. A system of regulated sale and treatment would also be implemented. The initiative provides that entities designated by a hospital that have received a “Certificate of Need” from the Michigan Department of Health and Human Services may administer and sell “Natural Plants and Mushrooms” to patients possessing a “written recommendation” for services from a Michigan licensed physician.
On June 1, 2022, activists behind the ballot initiative announced that the original target for inclusion on the ballot in 2022 has been deferred to 2024 to allow more time to gather the requisite signatures.
June 8, 2022
Colorado HB 22-1344 Signed Into Law
On March 28, 2022, HB 22-1344 was introduced into the House and assigned to the Public & Behavioral Health & Human Services committee. The bill states that if the United States food and drug administration approves a prescription medicine that contains 3,4-methylenedioxymethamphetamine (MDMA), and if that medicine has been placed on a schedule of the federal “Controlled Substances Act”, other than schedule I, or has been exempted from one or more provisions of such act, then thereafter prescribing, dispensing, transporting, possessing, and using that prescription drug is legal in Colorado in connection with MDMA-assisted therapy for PTSD and other comorbidities.
The bill passed the third reading in the House on April 8, 2022, and in the Senate on April 29, 2022. On June 8, 2022, Governor Jared Polis (D) signed HB 22-1344 into law.
June 13, 2022
Amherst (MA) Resolution Fails
On June 13th, 2022, the Amherst City Counsel failed to pass A Resolution Protecting Adult Access to Plant Medicines & Prioritizing Public Health Responses to Controlled Substance Possession, which would have deprioritized enforcement of “possession of controlled substances by adults, except the use of endangered plants and animal-derived controlled substances.” Though the resolution did not pass, several counsel members indicated support for decriminalization, but did not view such action within the scope of their authority.
June 23, 2022
New Jersey Bill S2934 Introduced
On June 23, 2022, Bill S2934 was introduced to the Senate and referred to Senate Health, Human Services and Senior Citizens Committees. The primary sponsor is Senator Nicholas Scutari (D). The “Psilocybin Behavioral Health Access and Services Act” would authorize production and use of psilocybin to promote health and wellness; decriminalizes, and expunges past offenses involving, psilocybin production, possession, use, and distribution.
July 1, 2022
Maryland SB 709 & HB 1367 Take Effect
On February 4, 2022, SB 709 was introduced and assigned to the Budget and Taxation and Finance committee. The bill establishes the Post–Traumatic Stress Disorder Alternative Therapies Fund as a special, nonlapsing fund, that is not subject to § 7–302 of the state finance and procurement article. The purpose of the fund is to support the department in studying the effectiveness of and improving access to alternative therapies for post–traumatic stress disorder in veterans. “Alternative therapies” includes hyperbaric oxygen therapy and psychedelics including 3,4–methylenedioxymethamphetamine (MDMA), psilocybin, and ketamine. The bill will take effect July 1, 2022.
On February 11, 2022, HB 1367 was introduced and assigned to the Appropriations committee. The bill mirrors SB 709 and will take effect July 1, 2022.
September 13, 2022
Hazel Park (MI) Resolution Passed
On September 13, 2022, the city council for the City of Hazel Park unanimously passed a resolution sponsored by councilmember Luke Londo, which designates September as “Entheogenic Plant and & Fungi Awareness Month.” Councilmember Londo stated in a recent interview discussing the designation that this “demonstrates our continued commitment to sensible policy, and hopefully signals to multiple cities in Michigan currently debating decriminalizing resolutions that there is widespread appeal and support for entheogenic plants and fungi.”
September 16, 2022
San Francisco (CA) Resolution Passed
On September, 16, 2022, the San Francisco Board of Supervisors passed a resolution that urges law enforcement officials to make “the investigation and arrest of individuals involved with the adult use of Entheogenic Plants on the Federal Schedule 1 List” among “the lowest priority for the City and County of San Francisco”. The resolution also urges the State of California and the federal government to decriminalize entheogenic plants.
October 3, 2022
New York Bill A6065 Reintroduced
On October 3, 2022, Assemblymember Linda B. Rosenthal reintroduced a heavily revised version of bill (A6065), which would have decriminalized psilocybin. The revised version of bill A6065 would remove state and local prohibitions on the “possession, use, cultivation, production, creation, analysis, gifting, exchange, or sharing by or between natural persons of twenty-one years of age or older of a natural plant or fungus-based hallucinogen,” including DMT, ibogaine, mescaline, psilocybin, and psilocyn.
Additionally, this bill would authorize “supervision, guidance, peers engaging in risk mitigation, religious, spiritual, or related supportive services with or without remuneration, by natural persons of twenty-one years of age or older to natural persons of twenty-one years of age or older who are engaging in the intentional and consenting use of natural plant or fungus-based hallucinogens.” The bill would also direct state and local law enforcement to refrain from assisting or cooperating with the government of the United States in the enforcement of the Controlled Substances Act relating to activities authorized under the bill, except as pursuant to a valid court order.
October 10, 2022
Atlanta (GA) Resolution 22-R-4257 Held For Further Consideration
On October 10, 2022, Atlanta City Council members decided not to advance the resolution; instead, it was determined by unanimous vote that the resolution would be held in committee for further consideration.
November 8, 2022
Colorado Prop 122 Passes
On November 8, 2022, the voters of Colorado passed Proposition 122, making Colorado the second state in the country to enact a regulated access program for psychedelics. Proposition 122 will establish the Natural Medicine Advisory Board which will advise Colorado regulators on the creation of state sanctioned “healing centers.” The proposition will also allow individuals over 21 years old to obtain access to “natural medicines” at “healing centers.” The proposition defines the term “natural medicine” to initially include only psilocybin and psilocyn. However, the term would be expanded on June 1, 2026, to include dimethyltryptamine, ibogaine, and mescaline (excluding peyote).
The Department of Regulatory Agencies (the Department) will have until January 1, 2024, to “establish the qualifications, education, and training requirements that facilitators must meet prior to providing natural medicine services.” Further, no later than September 30, 2024, “the Department shall adopt rules necessary to implement the regulated natural medicine access program and shall begin accepting applications for licensure by the date with decisions made on all licensing applications within 60 days of receiving the application.” The specific administrative rules that are passed in the coming years will be important because they will have a large impact on exactly what regulated access looks like in Colorado.
December 19, 2022
California SB 58 Introduced
On December 19, 2022, State Senator Scott Wiener introduced SB 58, which would legalize the possession, transportation, transfer, preparation, and obtaining allowable amounts of psilocybin, psilocyn, dimethyltryptamine (DMT), ibogaine and mescaline (excluding peyote) for personal or facilitated or supported use by persons 21 years old or older. The bill specifies that “allowable amount” means 2 grams of DMT, 15 grams of ibogaine, 2 grams of psilocybin or psilocin, or four ounces of plant or fungi containing psilocybin or psilocyn. This bill would also repeal the provision of the law that “prohibits the cultivation, transfer, or transportation, as specified, of any spores or mycelium capable of producing mushrooms or other material which contain psilocybin or psilocyn.”
Part of our Year in Review series
This content is part of our 2022 Year in Review, which looks back at the past year through commentary and analysis, interviews and guest contributions.
Receive New Sections in Your Inbox
To receive future sections of the Review in your inbox, join our newsletter…
- Though, this poses an entirely different set of issues: predominantly on the ethics and conflicts front.
- At a time when we were tracking states and localities at risk of opting out.
- The act effectively replaces the Right to Try Clarification Act.
- Though, it’s likely that the pair did not collaborate on their separate amendments.
- Interestingly, a couple of months before the DOJ investigation came to light, Cerebral partnered with Field Trip Health to “provide end-to-end mental health care”.
- “Restricted drugs are controlled substances regulated under Part J of the Food and Drug Regulations. They generally have no approved medical uses and can only be used for scientific or research purposes, such as clinical trials.”